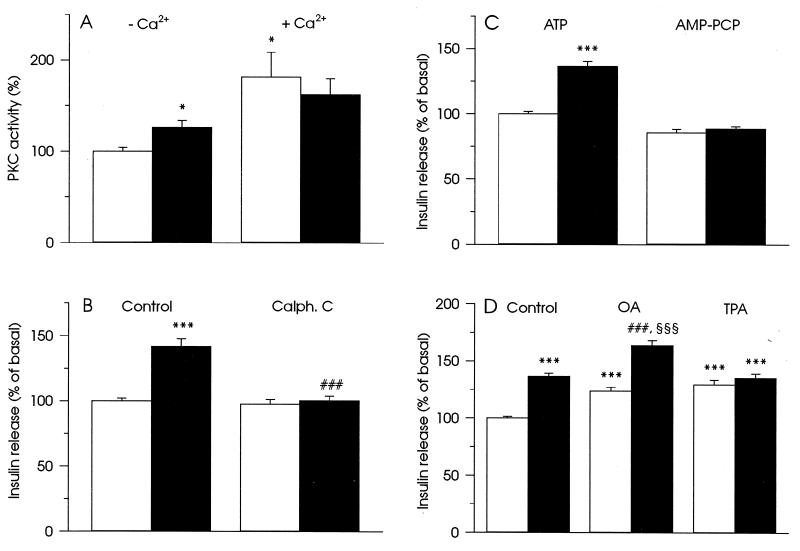
Figure 3.
Involvement of PKC activation in the stimulation of insulin secretion in HIT T15 cells by InsP6. (A) Modulation of PKC activity by 50 μM InsP6 (▪) in the absence and the presence of free Ca2+ as described in Materials and Methods. (□) The enzyme activity without InsP6. Data represent mean ± SEM of four experiments. ∗, P < 0.05, relative to PKC activity in the absence of free Ca2+ and InsP6. (B) Inhibitor of PKC, 1.5 μM Calphostin C (Calph. C), blocked increase in insulin secretion induced by 50 μM InsP6. (C) Requirement of ATP-hydrolysis for the development of the stimulatory effect of 50 μM InsP6 on insulin secretion. ATP (2 mM) was substituted by 2 mM AMP-PCP, a nonhydrolyzable analog of ATP. (D) Additive effect of 50 μM InsP6 and 1 μM okadaic acid (OA) on stimulation of insulin secretion and the absence of additive effect of 50 μM InsP6 and 100 nM TPA. In B–D insulin secretion was measured in buffer with basal Ca2+ concentration (30 nM). (□) Insulin secretion without InsP6 and black columns illustrate insulin secretion with 50 μM InsP6. The presence of other compounds is indicated in the figure. For measurements of insulin secretion, data represent mean ± SEM for 17 (B), 18 (C), and 20 (D) observations from three separate experiments. ∗∗∗, P < 0.001, relative to insulin secretion at 30 nM Ca2+. ###, P < 0.001, relative to insulin secretion in the presence of 50 μM InsP6. §§§, P < 0.001, relative to insulin secretion in the presence of 1 μM OA.