Abstract
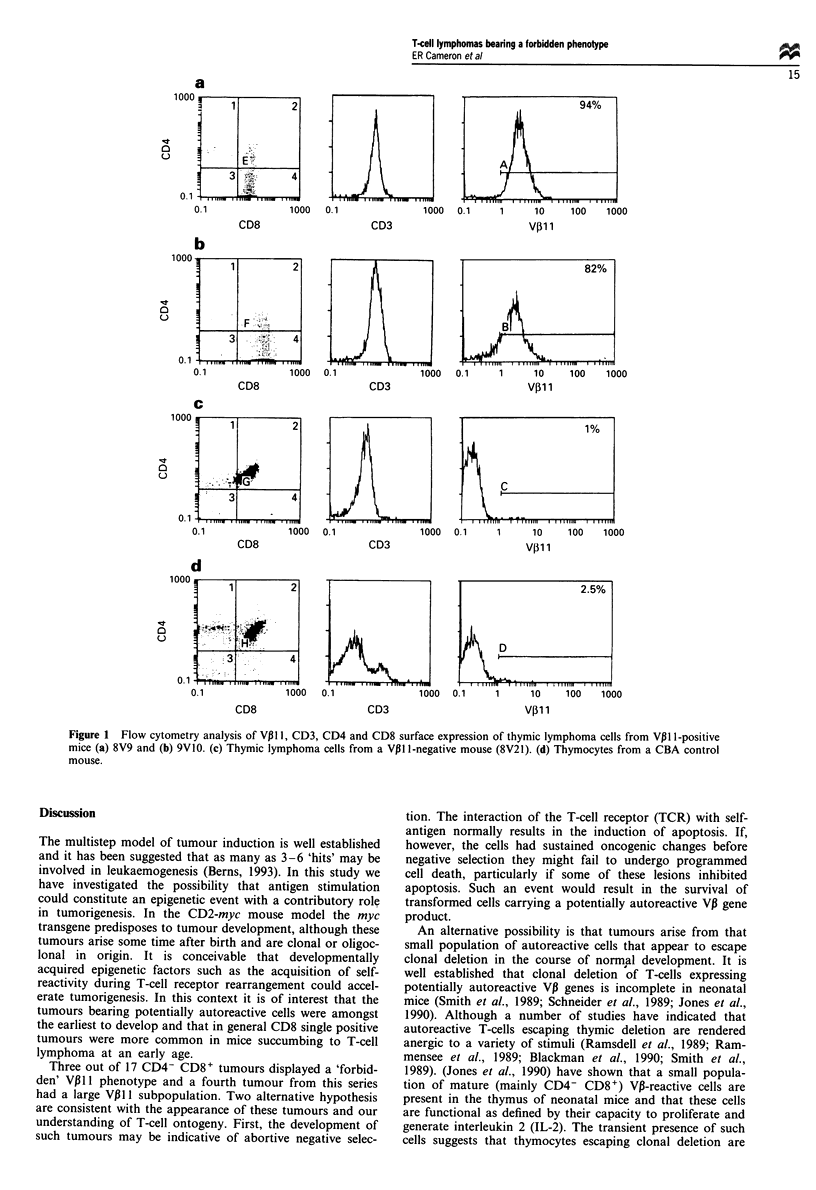
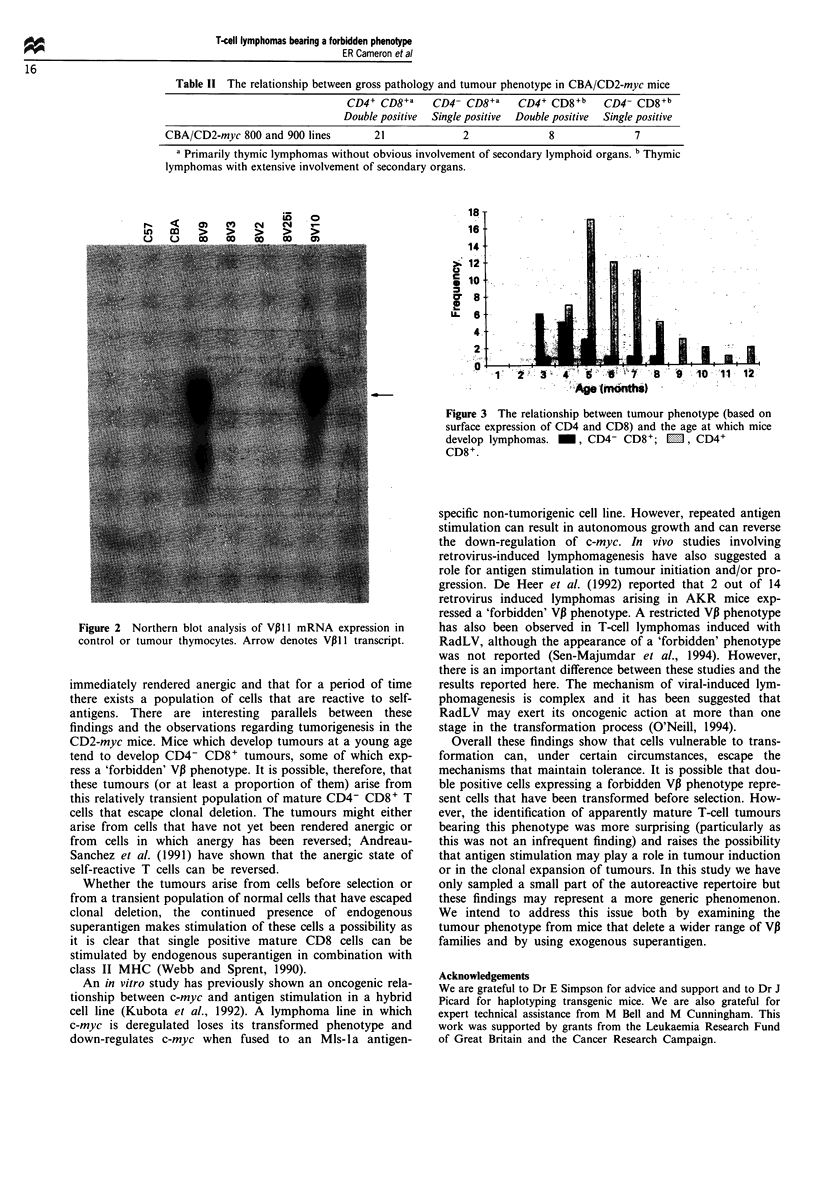
A role for antigen stimulation in lymphoid neoplasia has been postulated and is supported by indirect evidence that suggests that the interaction of antigen with both T cells and B cells may constitute an epigenetic event that can contribute to tumour induction or tumour progression. Using myc-bearing transgenic mice that develop mainly clonal T-cell lymphomas we have investigated the possibility that endogenous antigen-mediated clonal deletion might be overridden in tumorigenesis. CD2-myc transgenic mice were backcrossed on to a CBA/Ca background to ensure Mtv-mediated deletion of V beta 11-expressing T cells in the resultant offspring. Lymphomas arising from these mice were subsequently screened for V beta 11 expression. There was a clear correlation between the age at which mice developed neoplasia and the tumour phenotype. Mice with CD4- CD8+ tumours succumbed to thymic lymphoma at a significantly younger age than mice developing CD4+ CD8+ tumours. A small number of tumours consisted of the 'forbidden' V beta 11 phenotype, showing that cells vulnerable to transformation could escape negative selection. The majority of the V beta 11-positive tumours were CD4- CD8+ and were only observed in mice showing clinical evidence of tumour development at a relatively young age. The phenotype of these cells and the age at which tumours arose suggests that T cells escaping tolerance may be susceptible to transformation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Palmer E. Mls--a retrovirus exploits the immune system. Immunol Today. 1991 Oct;12(10):356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- Andreu-Sánchez J. L., Moreno de Alborán I. M., Marcos M. A., Sánchez-Movilla A., Martínez-A C., Kroemer G. Interleukin 2 abrogates the nonresponsive state of T cells expressing a forbidden T cell receptor repertoire and induces autoimmune disease in neonatally thymectomized mice. J Exp Med. 1991 Jun 1;173(6):1323–1329. doi: 10.1084/jem.173.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler D. W., Levy R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6770–6774. doi: 10.1073/pnas.89.15.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Burgert H. G., Gerhard-Burgert H., Woodland D. L., Palmer E., Kappler J. W., Marrack P. A role for clonal inactivation in T cell tolerance to Mls-1a. Nature. 1990 Jun 7;345(6275):540–542. doi: 10.1038/345540a0. [DOI] [PubMed] [Google Scholar]
- De Heer C., De Geus B., Schuurman H. J., Van Loveren H., Rozing J. V beta gene family usage in spontaneous lymphomas of AKR mice: evidence for defective clonal deletion. Dev Immunol. 1992;2(2):95–101. doi: 10.1155/1992/91527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero G., Hart S., Lim A., Borche L., Levy R., Miller R. A. Autoantibody activity of immunoglobulins isolated from B-cell follicular lymphomas. Blood. 1991 Aug 1;78(3):581–585. [PubMed] [Google Scholar]
- Fulton R., Forrest D., McFarlane R., Onions D., Neil J. C. Retroviral transduction of T-cell antigen receptor beta-chain and myc genes. Nature. 1987 Mar 12;326(6109):190–194. doi: 10.1038/326190a0. [DOI] [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Merriam G. R., Nelson L. M., Kruisbeck A. M. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J Exp Med. 1990 Nov 1;172(5):1277–1285. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K., Imreh S., Katoh H., Babonits M., Wiener F. Correlation of myc expression with the growth-arrested and transformed phenotypes in hybrids between a T lymphoma and an antigen-responsive T-cell line. Int J Cancer. 1992 Jul 30;51(6):927–934. doi: 10.1002/ijc.2910510616. [DOI] [PubMed] [Google Scholar]
- Marrack P., Winslow G. M., Choi Y., Scherer M., Pullen A., White J., Kappler J. W. The bacterial and mouse mammary tumor virus superantigens; two different families of proteins with the same functions. Immunol Rev. 1993 Feb;131:79–92. doi: 10.1111/j.1600-065x.1993.tb01531.x. [DOI] [PubMed] [Google Scholar]
- O'Neill H. C. Growth regulation of a T-cell lymphoma via the T-cell receptor. Leuk Res. 1994 Jan;18(1):23–28. doi: 10.1016/0145-2126(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Lantz T., Fowlkes B. J. A nondeletional mechanism of thymic self tolerance. Science. 1989 Nov 24;246(4933):1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- Schneider R., Lees R. K., Pedrazzini T., Zinkernagel R. M., Hengartner H., MacDonald H. R. Postnatal disappearance of self-reactive (V beta 6+) cells from the thymus of Mlsa mice. Implications for T cell development and autoimmunity. J Exp Med. 1989 Jun 1;169(6):2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen-Majumdar A., Weissman I. L., Hansteen G., Marian J., Waller E. K., Lieberman M. Radiation leukemia virus-induced thymic lymphomas express a restricted repertoire of T-cell receptor V beta gene products. J Virol. 1994 Feb;68(2):1165–1172. doi: 10.1128/jvi.68.2.1165-1172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E., Dyson P. J., Knight A. M., Robinson P. J., Elliott J. I., Altmann D. M. T-cell receptor repertoire selection by mouse mammary tumor viruses and MHC molecules. Immunol Rev. 1993 Feb;131:93–115. doi: 10.1111/j.1600-065x.1993.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Simpson E. Positive and negative selection of the T cell repertoire: role of MHC and other ligands. Int Rev Immunol. 1992;8(4):269–277. doi: 10.3109/08830189209053512. [DOI] [PubMed] [Google Scholar]
- Smith H., Chen I. M., Kubo R., Tung K. S. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989 Aug 18;245(4919):749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- Stewart M., Cameron E., Campbell M., McFarlane R., Toth S., Lang K., Onions D., Neil J. C. Conditional expression and oncogenicity of c-myc linked to a CD2 gene dominant control region. Int J Cancer. 1993 Apr 1;53(6):1023–1030. doi: 10.1002/ijc.2910530628. [DOI] [PubMed] [Google Scholar]
- Webb S. R., Sprent J. Response of mature unprimed CD8+ T cells to Mlsa determinants. J Exp Med. 1990 Mar 1;171(3):953–958. doi: 10.1084/jem.171.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]



