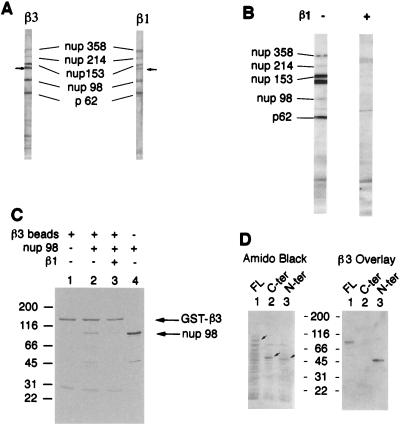
Figure 4.
Karyopherin β3 and karyopherin β1 compete for binding to repeat nucleoporins in overlay assay and in solution. (A) Nuclear envelope proteins were electrophoresed and transferred to nitrocellulose. Karyopherin β1 or β3 was added followed by the appropriate primary antibody and a secondary horseradish peroxidase-conjugated antibody. The signal was detected by chemiluminescence. The small arrows indicate a previously observed (3) unidentified band that interacts with both karyopherin β1 and β3. (B) Nuclear envelope protein blots were subjected to overlay assay with karyopherin β3 as in A but in the presence or absence of 10-fold molar excess of karyopherin β1 as indicated. The control lane (without karyopherin β1) contained glutathione elution buffer (see Materials and Methods) to control for the presence of this buffer in the karyopherin β1 preparation. The weak Nup98 signal in this lane may be due to the glutathione tripeptide competing with Nup98 for binding to karyopherin β3. (C) GST-karyopherin β3 fusion protein was bound to glutathione beads (lane 1), and Nup98 was added in the absence (lane 2) or presence (lane 3) of 5-fold molar excess of karyopherin β3. Lane 4 shows the Nup98 preparation in the absence of beads. (D) Bacterial lysates containing recombinant near-full length Nup98 (13) (lane 1), its C terminus (lane 2), or its repeat-containing N terminus (lane 3) were electrophoresed and transferred to nitrocellulose. The blot was stained with amido black (Left) and subjected to overlay assay with karyopherin β3 as in A (Right). Small arrows indicate the positions of the Nup98 bands in the amido black-stained gel.