Abstract
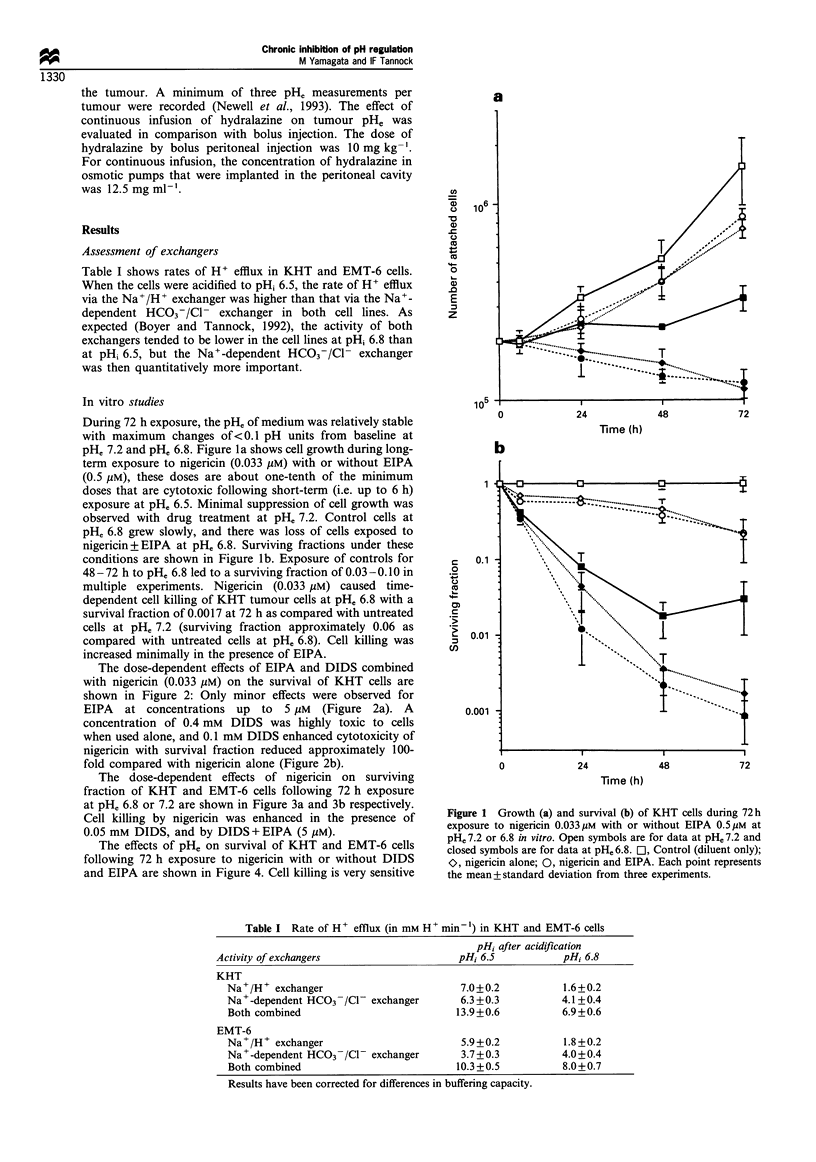
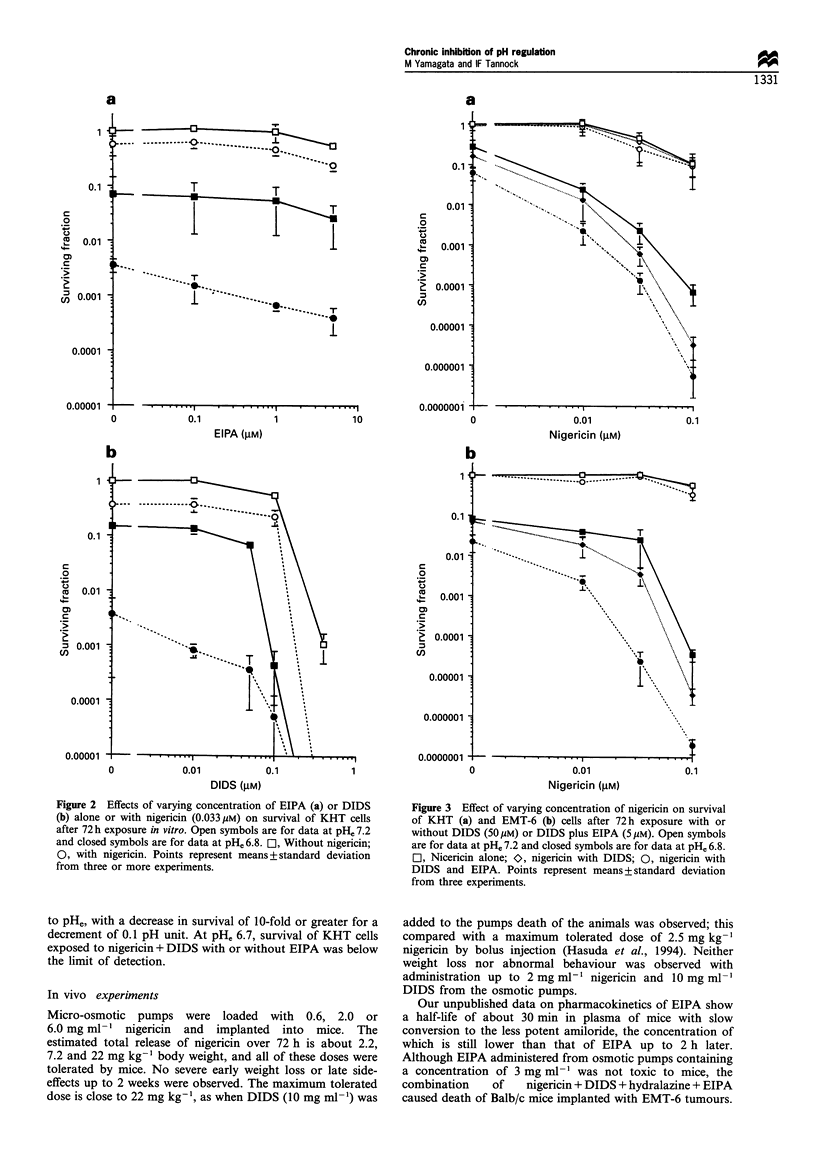
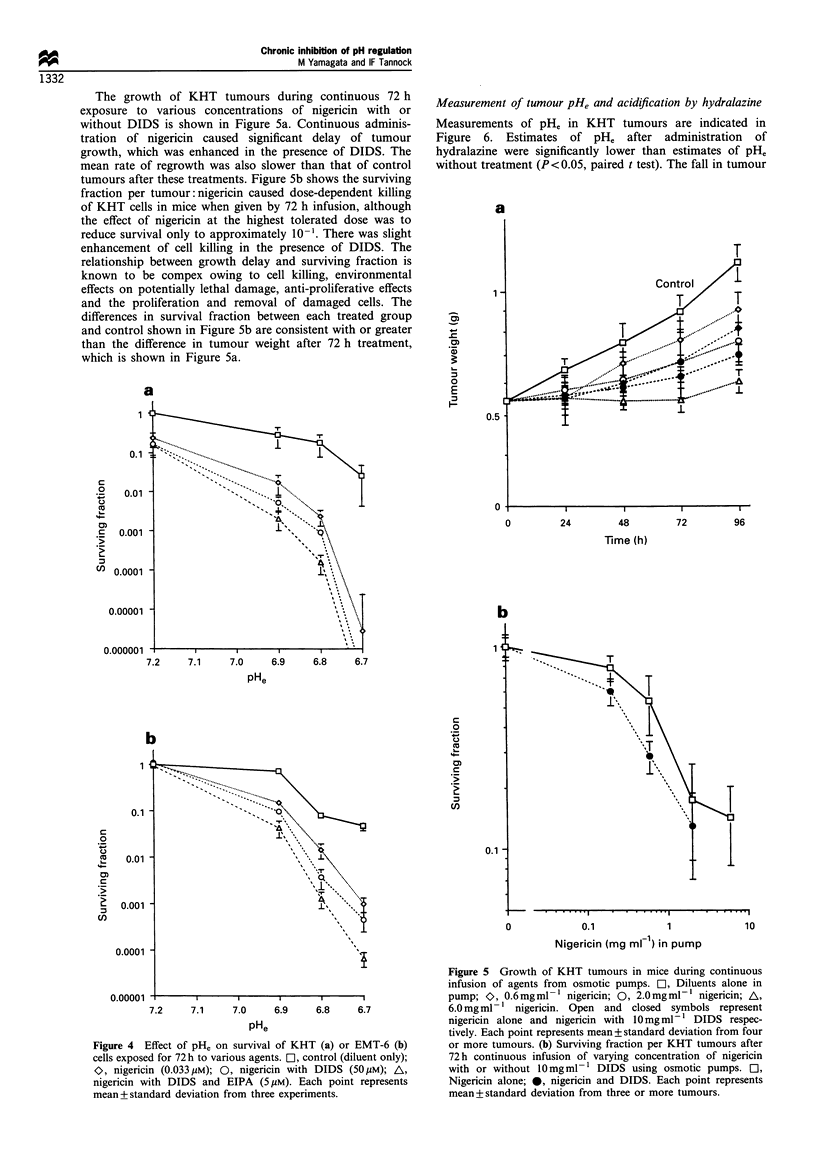
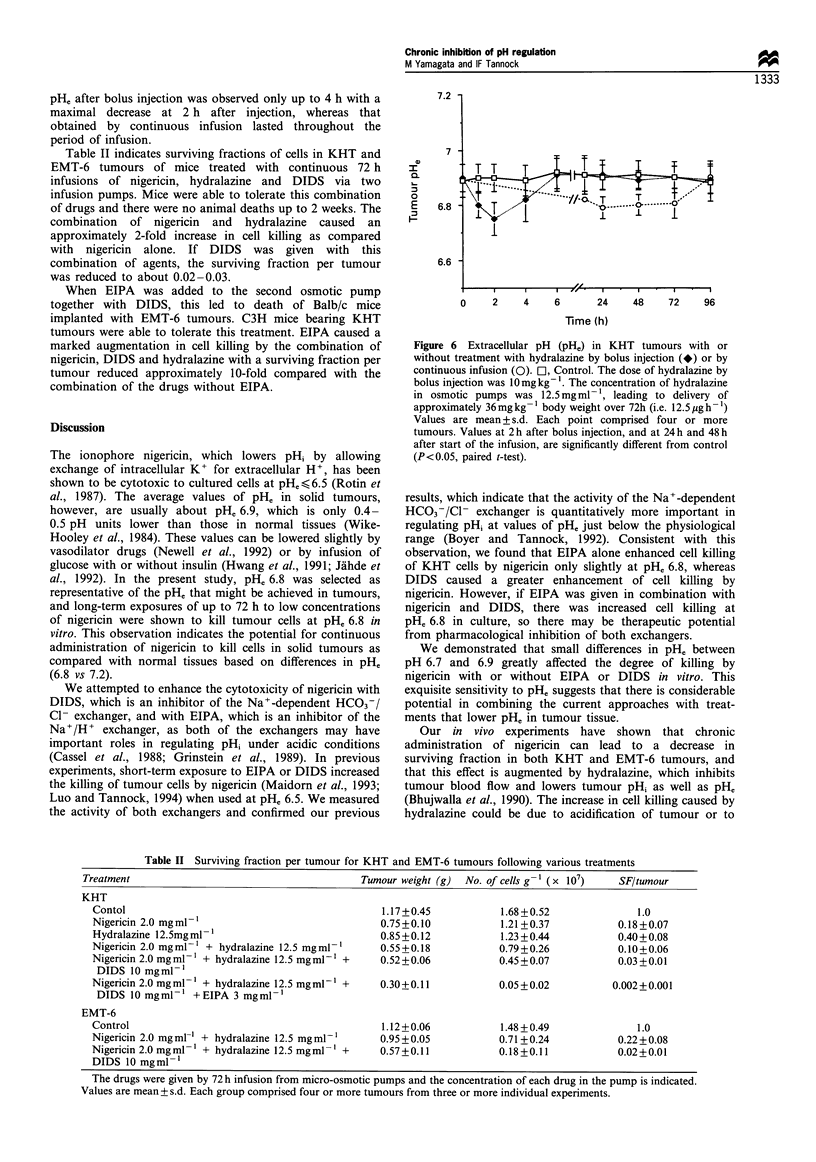
Mean values of extracellular pH (pHe) in tumours tend to be about 0.5 pH units lower than in normal tissues, whereas values of intracellular pH (pHi) in tumours and normal tissues are similar. Previous studies have shown that drugs that acidify cells at lower pHe such as nigericin, used alone or with agents that inhibit the regulation of pHi, have toxicity to cultured cells at pHe < 6.5 in short-term exposure; these agents also lead to modest anti-tumour effects in mice when given acutely. To evaluate the long-term effects of these drugs at levels of pHe that might occur commonly in tumours, we exposed cells for up to 72h at pHe 6.8 or 7.2 in vitro. Nigericin (0.033 microM) caused time-dependent cell killing of murine KHT and EMT-6 cells at pHe 6.8 (but not at pHe 7.2) with a surviving fraction approximately 5 x 10(-3) after 72 h exposure. Cell killing was increased in the presence of 4,4-diisothiocyanstilbene 2,2-disulphonic acid (DIDS), an inhibitor of Na+-dependent HCO3-/CI- exchange, and to a lesser extent in the presence of 5-(N-ethyl-N-isopropyl) amiloride (EIPA), an inhibitor of Na+/H+ exchange. Cell killing was exquisitely sensitive to the level of pHe. Osmotic pumps were used to obtain a 72 h continuous infusion of nigericin in mice; this led to dose-dependent killing of cells in KHT tumours with surviving fraction of approximately 0.1 at maximum tolerated doses. Hydralazine, which may cause tumour hypoxia and lower pHi as well as pHe, caused cytotoxity when given alone by chronic infusion, and enhanced the cytotoxicity due to nigericin. The addition of DIDS and/or EIPA (using two pumps) further enhanced anti-tumour toxicity, with a surviving fraction of approximately 0.002 at tolerated doses of the four drugs used to treat KHT tumours. The experiments demonstrate the activity of drugs that inhibit the regulation of pHi against murine tumours when delivered by chronic infusion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Bhujwalla Z. M., Tozer G. M., Field S. B., Maxwell R. J., Griffiths J. R. The energy metabolism of RIF-1 tumours following hydralazine. Radiother Oncol. 1990 Nov;19(3):281–291. doi: 10.1016/0167-8140(90)90155-p. [DOI] [PubMed] [Google Scholar]
- Boyer M. J., Tannock I. F. Regulation of intracellular pH in tumor cell lines: influence of microenvironmental conditions. Cancer Res. 1992 Aug 15;52(16):4441–4447. [PubMed] [Google Scholar]
- Cassel D., Scharf O., Rotman M., Cragoe E. J., Jr, Katz M. Characterization of Na+-linked and Na+-independent Cl-/HCO3- exchange systems in Chinese hamster lung fibroblasts. J Biol Chem. 1988 May 5;263(13):6122–6127. [PubMed] [Google Scholar]
- Gillies R. J., Liu Z., Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994 Jul;267(1 Pt 1):C195–C203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Hasuda K., Lee C., Tannock I. F. Antitumor activity of nigericin and 5-(N-ethyl-N-isopropyl)amiloride: an approach to therapy based on cellular acidification and the inhibition of regulation of intracellular pH. Oncol Res. 1994;6(6):259–268. [PubMed] [Google Scholar]
- Hwang Y. C., Kim S. G., Evelhoch J. L., Seyedsadr M., Ackerman J. J. Modulation of murine radiation-induced fibrosarcoma-1 tumor metabolism and blood flow in situ via glucose and mannitol administration monitored by 31P and 2H nuclear magnetic resonance spectroscopy. Cancer Res. 1991 Jun 15;51(12):3108–3118. [PubMed] [Google Scholar]
- Jähde E., Volk T., Atema A., Smets L. A., Glüsenkamp K. H., Rajewsky M. F. pH in human tumor xenografts and transplanted rat tumors: effect of insulin, inorganic phosphate, and m-iodobenzylguanidine. Cancer Res. 1992 Nov 15;52(22):6209–6215. [PubMed] [Google Scholar]
- Kim G. E., Lyons J. C., Song C. W. Effects of amiloride on intracellular pH and thermosensitivity. Int J Radiat Oncol Biol Phys. 1991 Mar;20(3):541–549. doi: 10.1016/0360-3016(91)90067-e. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Role of a Na+-dependent Cl-/HCO3- exchange in regulation of intracellular pH in fibroblasts. J Biol Chem. 1985 Apr 25;260(8):4877–4883. [PubMed] [Google Scholar]
- Lyons J. C., Ross B. D., Song C. W. Enhancement of hyperthermia effect in vivo by amiloride and DIDS. Int J Radiat Oncol Biol Phys. 1993 Jan;25(1):95–103. doi: 10.1016/0360-3016(93)90150-t. [DOI] [PubMed] [Google Scholar]
- Maidorn R. P., Cragoe E. J., Jr, Tannock I. F. Therapeutic potential of analogues of amiloride: inhibition of the regulation of intracellular pH as a possible mechanism of tumour selective therapy. Br J Cancer. 1993 Feb;67(2):297–303. doi: 10.1038/bjc.1993.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi J., Oda W., Hirata M., Fukuhori N., Inagaki C. Effects of amiloride on thermosensitivity of Chinese hamster cells under neutral and acidic pH. Cancer Res. 1986 Apr;46(4 Pt 1):1840–1843. [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. The regulation of cytoplasmic pH in human fibroblasts. J Biol Chem. 1984 Jun 25;259(12):7563–7569. [PubMed] [Google Scholar]
- Newell K., Franchi A., Pouysségur J., Tannock I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1127–1131. doi: 10.1073/pnas.90.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K., Wood P., Stratford I., Tannock I. Effects of agents which inhibit the regulation of intracellular pH on murine solid tumours. Br J Cancer. 1992 Aug;66(2):311–317. doi: 10.1038/bjc.1992.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins C. S., Chadwick J. A., Chaplin D. J. Enhancement of chlorambucil cytotoxicity by combination with flavone acetic acid in a murine tumour. Anticancer Res. 1994 Jul-Aug;14(4A):1603–1608. [PubMed] [Google Scholar]
- Rotin D., Steele-Norwood D., Grinstein S., Tannock I. Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res. 1989 Jan 1;49(1):205–211. [PubMed] [Google Scholar]
- Rotin D., Wan P., Grinstein S., Tannock I. Cytotoxicity of compounds that interfere with the regulation of intracellular pH: a potential new class of anticancer drugs. Cancer Res. 1987 Mar 15;47(6):1497–1504. [PubMed] [Google Scholar]
- Ruifrok A. C., Konings A. W. Effects of amiloride on hyperthermic cell killing of normal and thermotolerant mouse fibroblast LM cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Sep;52(3):385–392. doi: 10.1080/09553008714551861. [DOI] [PubMed] [Google Scholar]
- Song C. W., Lyons J. C., Griffin R. J., Makepeace C. M., Cragoe E. J., Jr Increase in thermosensitivity of tumor cells by lowering intracellular pH. Cancer Res. 1993 Apr 1;53(7):1599–1601. [PubMed] [Google Scholar]
- Sparks R. L., Pool T. B., Smith N. K., Cameron I. L. Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 1983 Jan;43(1):73–77. [PubMed] [Google Scholar]
- Szolgay-Daniel E., Carlsson J., Zierold K., Holtermann G., Dufau E., Acker H. Effects of amiloride treatment on U-118 MG and U-251 MG human glioma and HT-29 human colon carcinoma cells. Cancer Res. 1991 Feb 1;51(3):1039–1044. [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]


