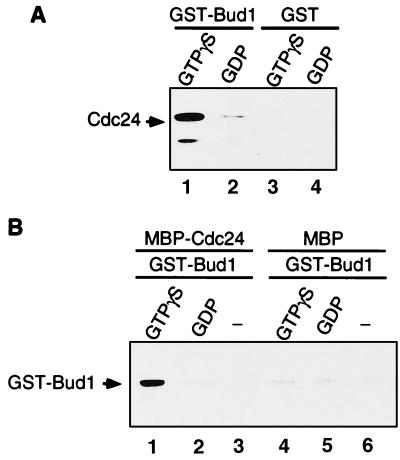
Figure 1.
Cdc24 binds preferentially to GTP[γS]-bound Bud1 in vitro. (A) Association of GST–Bud1 and six-histidine-tagged Cdc24. Purified GST–Bud1 and GST (final concentration, approximately 750 nM) were preincubated with 1 mM GDP or GTP[γS] and then incubated with six-histidine-tagged Cdc24 (final concentration, 15 nM). Cdc24 was incubated with either GTP[γS]-bound Bud1 (lane 1) or GDP-bound Bud1 (lane 2), or with GST that had been preincubated with GTP[γS] (lane 3) or GDP (lane 4). Proteins bound to glutathione-Sepharose beads were collected and analyzed by SDS/PAGE. Approximately equal amounts of GST–Bud1 and GST proteins were recovered for each reaction as judged by Coomassie blue staining of the gel (data not shown). Association of Cdc24 was determined by immunoblotting with polyclonal antibodies against Cdc24. (B) Association of GST–Bud1 and MBP–Cdc24 in vitro. MBP–Cdc24 (750 nM) was incubated either with GST–Bud1 (75 nM) preloaded with GTP[γS] (lane 1) or GDP (lane 2), or with GST–Bud1 that had not been preincubated with any nucleotides (lane 3). MBP (750 nM) was also incubated with GST–Bud1 (75 nM) preloaded either with GTP[γS] (lane 4) or GDP (lane 5), or with GST–Bud1 that had not been preincubated with any nucleotides (lane 6). Proteins bound to amylose resin were collected and analyzed by SDS/PAGE. Approximately equal amounts of MBP–Cdc24 and MBP were recovered for each reaction as judged by Coomassie blue staining of the gel (data not shown). Association of GST–Bud1 was determined by immunoblotting with polyclonal antibodies against GST.