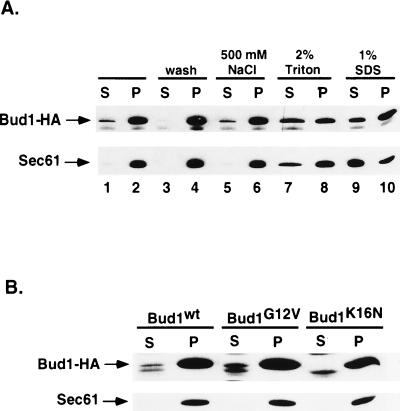
Figure 4.
Subcellular fractionation and solubilization of Bud1 from the particulate fraction. (A) Subcellular fractionation and solubilization of wild-type Bud1. Cells of yeast strain expressing HA-epitope-tagged Bud1 were broken open by bead beating and centrifuged at 500 × g to produce whole cell extract (wce); the wce was subsequently centrifuged at 10,000 × g to produce pellect (P) and supernatant (S) fractions (lanes 1 and 2). Aliquots of the pellet fractions (P) were washed with the same lysis buffer (lanes 3 and 4) or treated with 500 mM NaCl (lanes 5 and 6), 2% Triton (lanes 7 and 8), and 1% SDS (lanes 9 and 10) before centrifuging at 10,000 × g again (34). Both pellet and supernatant fractions from equal amounts of cells (OD600 unit) were loaded on 10% polyacrylamide gel and subjected to immunoblot analysis using monoclonal antibodies against HA epitope to detect HA-tagged Bud1. As a control for fractionation, identical samples were also subjected to immunoblot analysis using antibodies against the yeast endoplasmic reticulum membrane protein Sec61 (36). (B) Subcellular fractionation of Bud1G12V and Bud1K16N. Cells of yeast strain expressing HA-epitope-tagged Bud1G12V or Bud1K16N were treated in the same way as described in A.