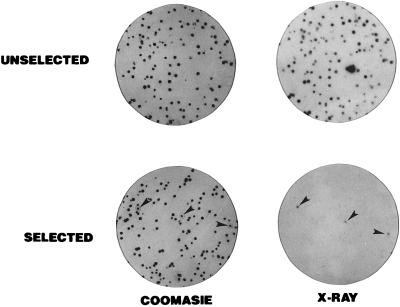
Figure 1.
Screening of the P9OH/UV-resistant population for peroxisomal DHAP-ATase activity by colony autoradiography. Cells were plated out into 100-mm-diameter tissue culture dishes at a concentration of 400 cells/dish and allowed to attach overnight. The cells were overlaid with a sterile polyester cloth (17) and left undisturbed at 37°C for 9 days to allow for the formation of colonies of cells both on the master dish and the polyester. The polyesters were removed, rinsed three times in 100 ml ice-cold PBS, and placed at −80°C to lyse the cells. The master dishes were fed with fresh medium and placed at 28°C to keep the colonies viable. Each polyester was thawed and placed in 3 ml of a solution containing 100 mM N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid, 100 mM Mes (pH 5.5), 100 μM palmitoyl-CoA, 1.5 mM [32P]DHAP (4–6 μCi/μmol), 8 mM NaF, 5 mM MgCl2, 50 mM KCl, 2 mM KCN, and 2 mg/ml bovine serum albumin (2, 8). After 15 min at 40°C, 3 ml 20% trichloroacetic acid (TCA) was added to precipitate the radioactive product (1-acyl-DHAP). The polyesters were washed three times with 50 ml 3% TCA and exposed to GBX-2 x-ray film following preflash. Following autoradiography, the colonies were visualized by staining with Coomassie blue (18). All of the colonies from the unselected population were DHAPAT+, as indicated by the corresponding signal on the x-ray film. Only three colonies yielded a signal in the P9OH/UV-selected population.