Abstract
We have analysed the expression of interleukin-2 receptor (IL-2R) on a panel of small-cell lung cancer (SCLC) cell lines. None of the 11 SCLC cell lines studied expressed detectable surface IL-2R alpha or beta chains by indirect immunofluorescence. Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses indicated that only one out of 11 cell lines expressed detectable IL-2R beta mRNA while two expressed a weak positivity for IL-2R gamma. Five SCLC cell lines were transfected with the plasmid vector RSV.5 neo containing IL-2 cDNA coding sequence. Stable transfectants secreted biologically active IL-2 (ranging from 25 to 100 U ml-1 in the culture supernatant). IL-2 transfection did not produce significant modifications in the expression of surface molecules such as IL-2R alpha and beta chains, intercellular adhesion molecule-1 (ICAM-1), CD44, HLA class I and II or in IL-2R beta or gamma mRNA. More importantly, IL-2-transfected N592 and NCI H69 cell lines completely lost their tumorigenic potential in nude mice after subcutaneous injection, whereas experimental controls transfected with RSV.5 neo vector only, displayed an in vivo growth pattern identical to that of untransfected cells. In addition, in the N592 model, IL-2-producing N592 inhibited the growth of wild-type N592 injected at the same site, while injection of parental cells on the opposite side did not significantly affect the growth of wild-type tumour cells. Histopathological analysis of the rejection process of IL-2-transfected cells demonstrated the presence of MAC-1+, MAC-3+ macrophages and of RB68C5+ granulocytes, whereas T cells were undetectable and NK cells were scarcely represented. In addition, a reduction of the tumour blood vessels was observed. The possible relevance of these data for the development of vaccination strategies using cytokine-engineered tumour cells in SCLC is discussed.
Full text
PDF

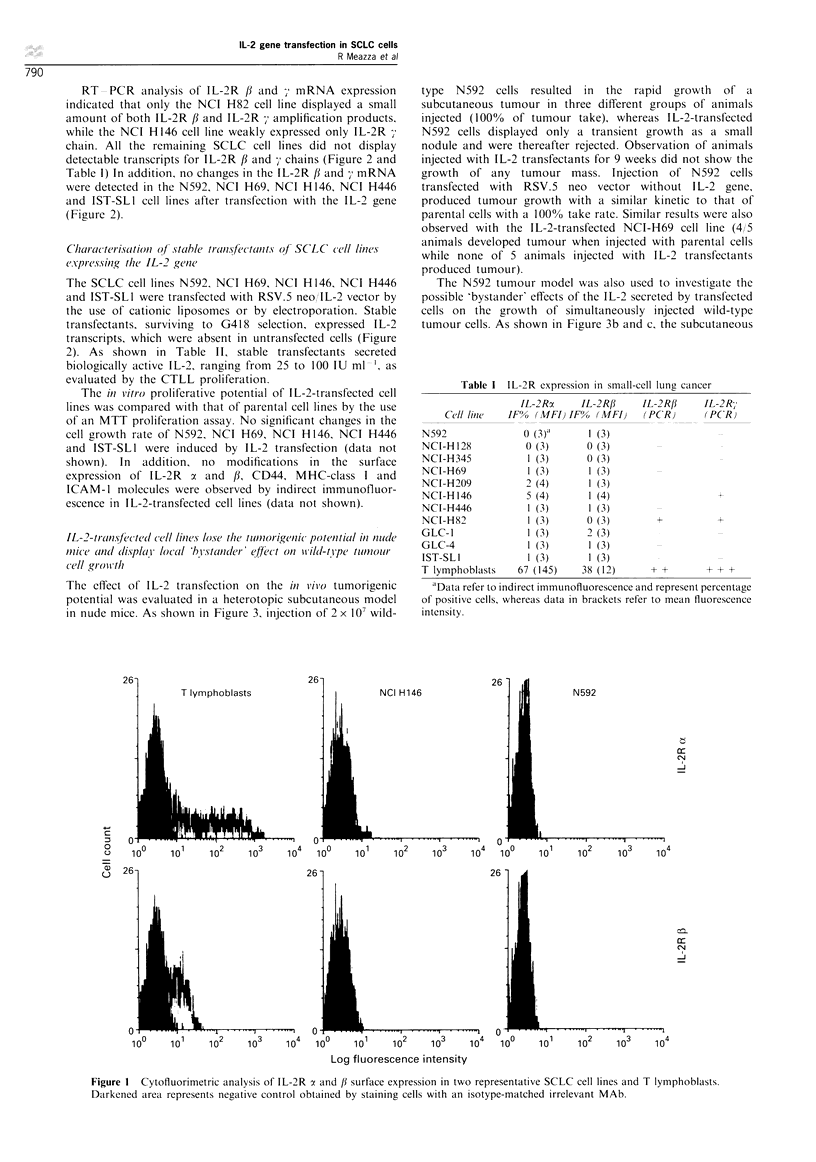
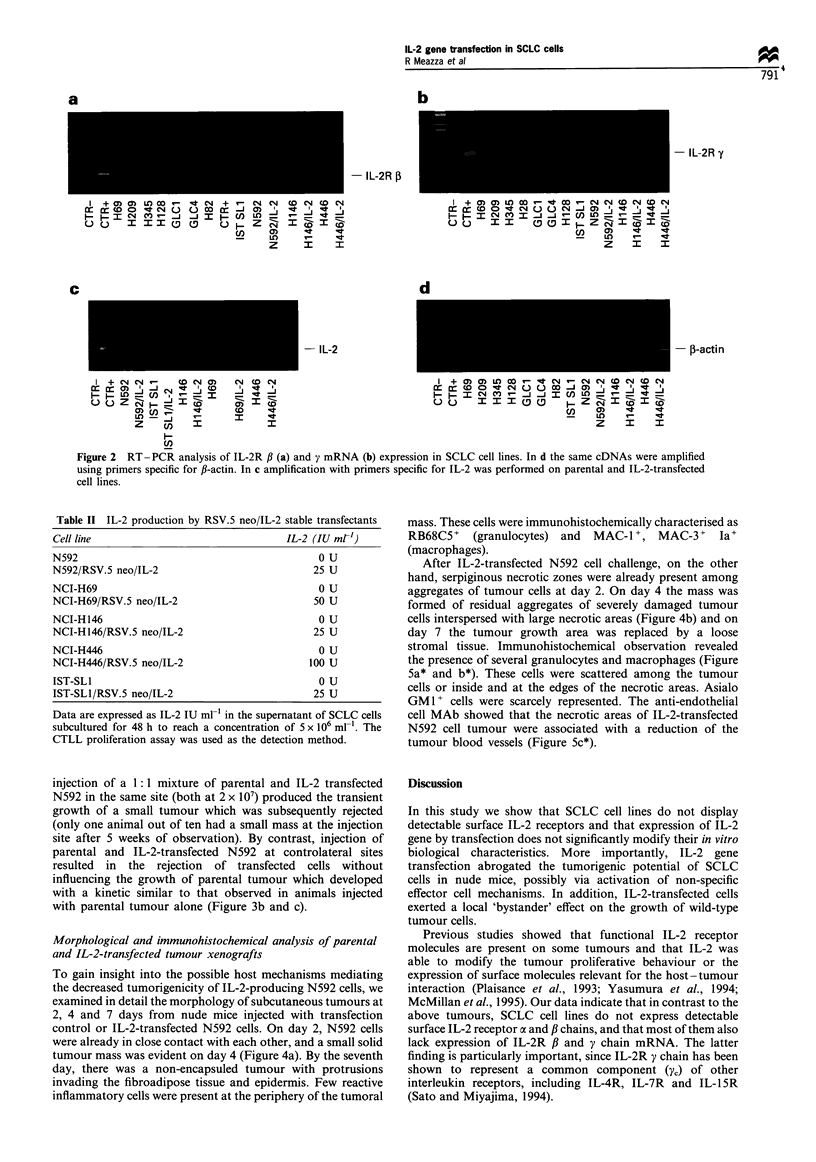
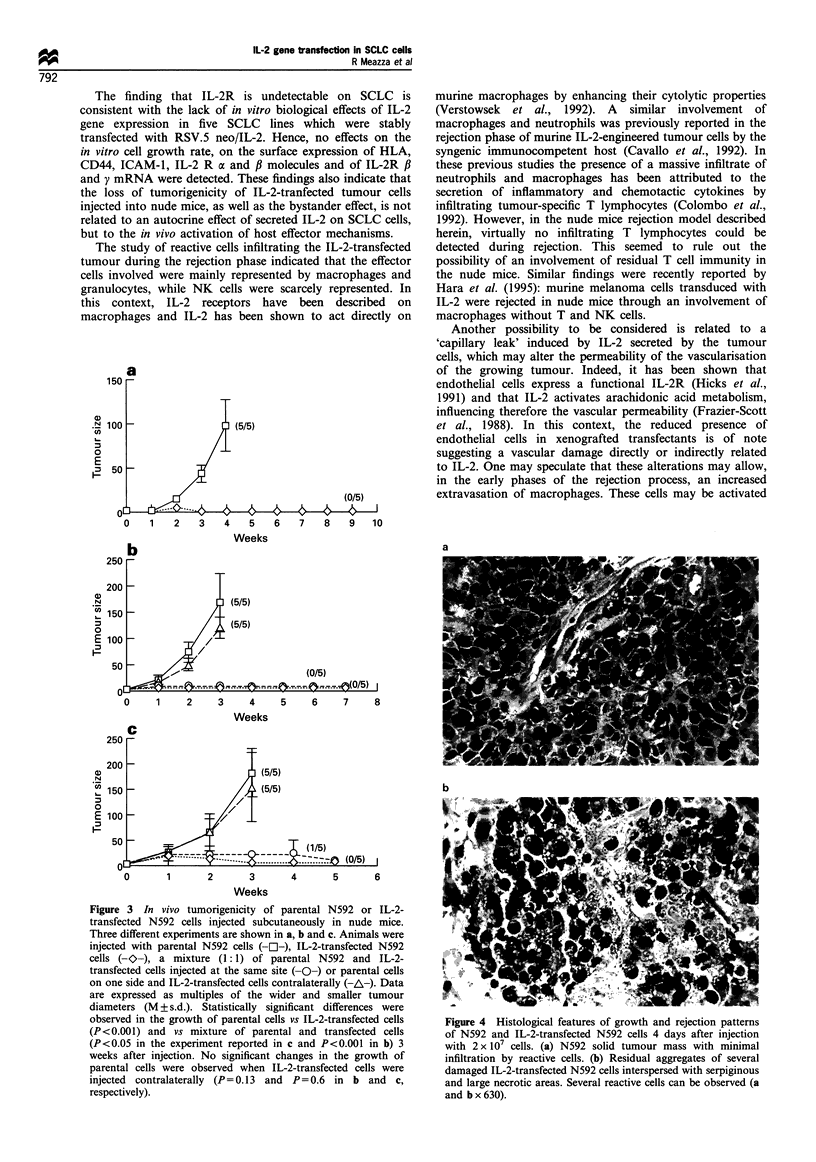


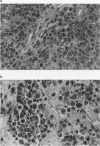
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Wahab Z., Li W. P., Osanto S., Darrow T. L., Hessling J., Vervaert C. E., Burrascano M., Barber J., Seigler H. F. Transduction of human melanoma cells with interleukin-2 gene reduces tumorigenicity and enhances host antitumor immunity: a nude mouse model. Cell Immunol. 1994 Nov;159(1):26–39. doi: 10.1006/cimm.1994.1292. [DOI] [PubMed] [Google Scholar]
- Blankenstein T., Qin Z. H., Uberla K., Müller W., Rosen H., Volk H. D., Diamantstein T. Tumor suppression after tumor cell-targeted tumor necrosis factor alpha gene transfer. J Exp Med. 1991 May 1;173(5):1047–1052. doi: 10.1084/jem.173.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassileth P. A., Podack E., Sridhar K., Savaraj N., Hanlon J. Phase I study of transfected cancer cells expressing the interleukin-2 gene product in limited stage small cell lung cancer. Hum Gene Ther. 1995 Mar;6(3):369–383. doi: 10.1089/hum.1995.6.3-369. [DOI] [PubMed] [Google Scholar]
- Cavallo F., Giovarelli M., Gulino A., Vacca A., Stoppacciaro A., Modesti A., Forni G. Role of neutrophils and CD4+ T lymphocytes in the primary and memory response to nonimmunogenic murine mammary adenocarcinoma made immunogenic by IL-2 gene. J Immunol. 1992 Dec 1;149(11):3627–3635. [PubMed] [Google Scholar]
- Clamon G., Herndon J., Perry M. C., Ozer H., Kreisman H., Maher T., Ellerton J., Green M. R. Interleukin-2 activity in patients with extensive small-cell lung cancer: a phase II trial of Cancer and Leukemia Group B. J Natl Cancer Inst. 1993 Feb 17;85(4):316–320. doi: 10.1093/jnci/85.4.316. [DOI] [PubMed] [Google Scholar]
- Colombo M. P., Forni G. Cytokine gene transfer in tumor inhibition and tumor therapy: where are we now? Immunol Today. 1994 Feb;15(2):48–51. doi: 10.1016/0167-5699(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Colombo M. P., Modesti A., Parmiani G., Forni G. Local cytokine availability elicits tumor rejection and systemic immunity through granulocyte-T-lymphocyte cross-talk. Cancer Res. 1992 Sep 15;52(18):4853–4857. [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Ferrini S., Miescher S., Zocchi M. R., von Fliedner V., Moretta A. Phenotypic and functional characterization of recombinant interleukin 2 (rIL 2)-induced activated killer cells: analysis at the population and clonal levels. J Immunol. 1987 Feb 15;138(4):1297–1302. [PubMed] [Google Scholar]
- Frasier-Scott K., Hatzakis H., Seong D., Jones C. M., Wu K. K. Influence of natural and recombinant interleukin 2 on endothelial cell arachidonate metabolism. Induction of de novo synthesis of prostaglandin H synthase. J Clin Invest. 1988 Dec;82(6):1877–1883. doi: 10.1172/JCI113805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler B., Van den Eynde B., van der Bruggen P., Romero P., Gaforio J. J., De Plaen E., Lethé B., Brasseur F., Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994 Mar 1;179(3):921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara I., Nguyen H., Takechi Y., Gansbacher B., Chapman P. B., Houghton A. N. Rejection of mouse melanoma elicited by local secretion of interleukin-2: implicating macrophages without T cells or natural killer cells in tumor rejection. Int J Cancer. 1995 Apr 10;61(2):253–260. doi: 10.1002/ijc.2910610219. [DOI] [PubMed] [Google Scholar]
- Hicks C., Cooley M. A., Penny R. Investigation of interleukin 2 receptors on human endothelial cells. Growth Factors. 1991;5(3):201–208. doi: 10.3109/08977199109000284. [DOI] [PubMed] [Google Scholar]
- Long E. O., Rosen-Bronson S., Karp D. R., Malnati M., Sekaly R. P., Jaraquemada D. Efficient cDNA expression vectors for stable and transient expression of HLA-DR in transfected fibroblast and lymphoid cells. Hum Immunol. 1991 Aug;31(4):229–235. doi: 10.1016/0198-8859(91)90092-n. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- McMillan D. N., Kernohan N. M., Flett M. E., Heys S. D., Deehan D. J., Sewell H. F., Walker F., Eremin O. Interleukin 2 receptor expression and interleukin 2 localisation in human solid tumor cells in situ and in vitro: evidence for a direct role in the regulation of tumour cell proliferation. Int J Cancer. 1995 Mar 16;60(6):766–772. doi: 10.1002/ijc.2910600606. [DOI] [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Plaisance S., Rubinstein E., Alileche A., Han D. S., Sahraoui Y., Mingari M. C., Bellomo R., Rimoldi D., Colombo M. P., Jasmin C. Human melanoma cells express a functional interleukin-2 receptor. Int J Cancer. 1993 Aug 19;55(1):164–170. doi: 10.1002/ijc.2910550129. [DOI] [PubMed] [Google Scholar]
- Rimoldi D., Salvi S., Hartmann F., Schreyer M., Blum S., Zografos L., Plaisance S., Azzarone B., Carrel S. Expression of IL-2 receptors in human melanoma cells. Anticancer Res. 1993 May-Jun;13(3):555–564. [PubMed] [Google Scholar]
- Rosenthal F. M., Cronin K., Bannerji R., Golde D. W., Gansbacher B. Augmentation of antitumor immunity by tumor cells transduced with a retroviral vector carrying the interleukin-2 and interferon-gamma cDNAs. Blood. 1994 Mar 1;83(5):1289–1298. [PubMed] [Google Scholar]
- Sato N., Miyajima A. Multimeric cytokine receptors: common versus specific functions. Curr Opin Cell Biol. 1994 Apr;6(2):174–179. doi: 10.1016/0955-0674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wolf G., Schmidt-Wolf I. G. Cytokines and clinical gene therapy. Eur J Immunol. 1995 Apr;25(4):1137–1140. doi: 10.1002/eji.1830250445. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Namikawa R. Species-specific metastasis of human tumor cells in the severe combined immunodeficiency mouse engrafted with human tissue. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4661–4665. doi: 10.1073/pnas.92.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversari C., van der Bruggen P., Luescher I. F., Lurquin C., Chomez P., Van Pel A., De Plaen E., Amar-Costesec A., Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992 Nov 1;176(5):1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. L., Shepard H. M., Rothstein J. L., Sugarman B. J., Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi A., Garlanda C., Lampugnani M. G., Resnati M., Matteucci C., Stoppacciaro A., Schnurch H., Risau W., Ruco L., Mantovani A. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994 Apr;63(2):247–254. [PubMed] [Google Scholar]
- Verstovsek S., Maccubbin D., Ehrke M. J., Mihich E. Tumoricidal activation of murine resident peritoneal macrophages by interleukin 2 and tumor necrosis factor alpha. Cancer Res. 1992 Jul 15;52(14):3880–3885. [PubMed] [Google Scholar]
- Voss S. D., Sondel P. M., Robb R. J. Characterization of the interleukin 2 receptors (IL-2R) expressed on human natural killer cells activated in vivo by IL-2: association of the p64 IL-2R gamma chain with the IL-2R beta chain in functional intermediate-affinity IL-2R. J Exp Med. 1992 Aug 1;176(2):531–541. doi: 10.1084/jem.176.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann E., Sacchi M., Plaisance S., Heo D. S., Yasumura S., Lin W. C., Johnson J. T., Herberman R. B., Azzarone B., Whiteside T. L. Receptors for interleukin 2 on human squamous cell carcinoma cell lines and tumor in situ. Cancer Res. 1992 Nov 1;52(21):5963–5970. [PubMed] [Google Scholar]
- Yasumura S., Lin W. C., Weidmann E., Hebda P., Whiteside T. L. Expression of interleukin 2 receptors on human carcinoma cell lines and tumor growth inhibition by interleukin 2. Int J Cancer. 1994 Oct 15;59(2):225–234. doi: 10.1002/ijc.2910590215. [DOI] [PubMed] [Google Scholar]
- de Leij L., Postmus P. E., Buys C. H., Elema J. D., Ramaekers F., Poppema S., Brouwer M., van der Veen A. Y., Mesander G., The T. H. Characterization of three new variant type cell lines derived from small cell carcinoma of the lung. Cancer Res. 1985 Dec;45(12 Pt 1):6024–6033. [PubMed] [Google Scholar]