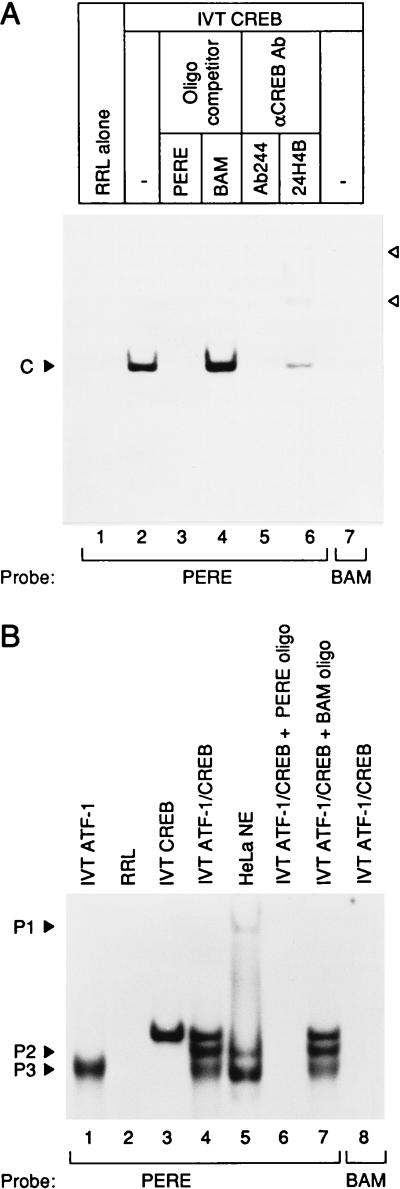
Figure 1.
CREB transcription factor binds to the PERE. (A) Full-length CREB was synthesized in vitro and incubated with α-32P-labeled PERE or BAM probe. IVT-CREB formed a specific complex (C) on the PERE probe not present with the mutant BAM probe. PERE probe was also incubated with unprogrammed reticulocyte lysate (RRL) as a control. Supershifted CREB complexes produced by anti-CREB antibodies are denoted by arrowheads. (B) CREB forms a heterodimer with ATF-1 on the PERE and forms complexes comigrating with complexes P2 and P3. Full-length ATF-1 and CREB were synthesized in vitro and bound to the PERE probe individually or mixed together in DNA binding reactions. The positions of P1–3, DNA-protein complexes formed from HeLa cell nuclear extract (5 μg), are indicated. The PERE oligonucleotide (5′-CAGCGTGGTGACGTCGCAACG-3′) contains sequences from −60 to −40 relative to the transcription start site of the human PCNA promoter. The BAM oligonucleotide (5′-CAGCGTGGTGGATCCGCAACG-3′) harbors a 4-bp mutation within the ATF consensus binding site that abrogates PCNA promoter transactivation by E1A in vivo (30, 32). Anti-CREB antibodies (Ab244, M. Montminy, Harvard Medical School; 24HB4, Santa Cruz Biotechnology) were preincubated in the DNA binding mixture for at least 1 hr at 4°C before the addition of radiolabeled probe.