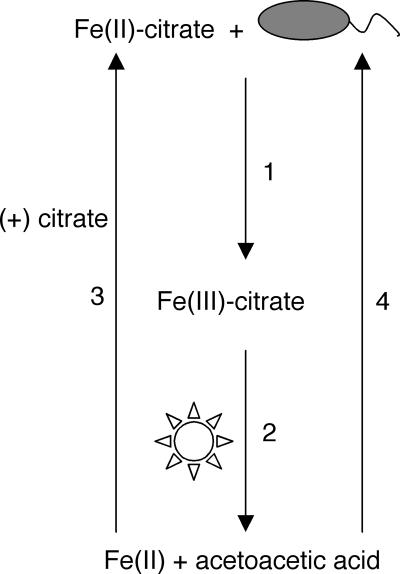
FIG. 4.
Model for Fe(II) oxidation leading to carbon acquisition. In the presence of Fe(II)-citrate, R. capsulatus SB1003 is able to oxidize Fe(II) leading to the formation of Fe(III)-citrate (arrow 1). In the presence of light, Fe(III)-citrate undergoes a photochemical reaction yielding Fe(II) and β-ketoglutarate and the latter spontaneously decarboxylates into acetoacetic acid (arrow 2). As a result of the photochemical reaction, the resulting Fe(II) can become bound by citrate (arrow 3) and microbially reoxidized. The acetoacetic acid that results from these reactions can be used as a carbon source for growth (arrow 4).