Abstract
Houseflies (Musca domestica) released into rooms containing hens challenged with Salmonella enterica serovar Enteritidis (Salmonella serovar Enteritidis) rapidly became contaminated with Salmonella serovar Enteritidis. Forty to 50% of the flies were contaminated at 48 h, and the percentage increased to 50 to 70% at 4 and 7 days postexposure and then decreased to 30% at day 15. Initial attempts at recovering surface organisms for culture using an aqueous rinse were largely unsuccessful, while cultures of internal contents readily recovered Salmonella serovar Enteritidis. However, when 0.5% detergent was incorporated into the rinse, high recovery levels of bacteria were observed from both external and internal culture regimens, indicating equal distribution of the organism on and in the fly and a tighter interaction of the organism with the host than previously thought. Salmonella serovar Enteritidis was isolated routinely from the fly gut, on rare occasions from the crop, and never from the salivary gland. Feeding contaminated flies to hens resulted in gut colonization of a third of the birds, but release of contaminated flies in a room containing previously unchallenged hens failed to result in colonization of any of the subject birds. These results indicate that flies exposed to an environment containing Salmonella serovar Enteritidis can become colonized with the organism and might serve as a source for transmission of Salmonella serovar Enteritidis within a flock situation.
Salmonella enterica serovar Enteritidis (Salmonella serovar Enteritidis) remains a serious food-borne threat to humans within the U.S. and overseas (6, 30). Poultry and their products constitute a significant proportion of the sources implicated in food-borne Salmonella outbreaks (30, 34, 36), prompting more rigorous focus by regulatory agencies and industry on implementing measures to reduce the incidence of these problem organisms on the farm and during processing. Many risk factors for exacerbating Salmonella infection in flocks have been identified on the farm, and animal vectors, both vertebrate and invertebrate, have been implicated in this role (13, 14, 37). Insects have long been associated with the spread of pathogens in human disease outbreaks (1, 9), and similar observations have been reported for poultry. Cockroaches (24), beetles (25, 33), and flies (10, 25, 29) recovered from poultry houses have all been reported to harbor Salmonella or other human pathogens, and chicken-to-chicken transmission of these organisms has also been observed (32).
Flies comprise a large and complex fauna of arthropods with worldwide distribution. Because of their intimate relationship with decaying matter, garbage, and feces, flies have long been associated with the potential for spreading disease. A. R. Olsen (28) lists 47 fly species from which Escherichia coli, an indicator of fecal contamination, have been isolated. Seventeen of these were found to carry the human pathogens Salmonella or Shigella and, of these, 14 were considered “communicative,” meaning that they moved between contaminated environments and interacted with man (8, 28). The housefly (Musca domestica) was a prominent member of this group and received the most citations regarding contamination with human pathogens. With regard to poultry, while a number of studies examined flies as carriers of Salmonella, no work has focused on the kinetics of colonization of flies with Salmonella upon exposure to a contaminated environment or where this organism resides on or in its arthropod host. The current study examined the time frame for fly contamination upon release into a room containing hens infected with Salmonella serovar Enteritidis, the location of the organism on or in the fly, and whether these contaminated flies could transmit Salmonella to naive nonstressed and stressed hens.
MATERIALS AND METHODS
Chickens.
Single-comb white leghorn chickens >60 weeks of age were obtained from the specific-pathogen-free flock maintained at the Southeast Poultry Research Laboratory (SEPRL), Athens, GA. Twenty-six, 24, and 26 hens were used in experiments 1, 2, and 3, respectively. The hens were transferred to individual, adjacent laying cages in an environmentally controlled biosafety level 2 building at SEPRL and allowed to acclimate for 7 to 4 days. The hens were fed layer rations ad libitum throughout the duration of the experiment. To ensure that the hens were Salmonella-free, the individuals were screened for Salmonella prior to the commencement of the experiments by enriching 1 g of feces in 9 ml of Rappaport-Vassiliadis (RV) enrichment medium (Oxoid, Inc., Basingstoke, England), which was incubated overnight at 37°C, after which 100 μl of the broth was plated onto XLT4 agar (Remel, Lenexa, KS). Salmonella was not detected. The studies were approved by and conducted under the guidelines of the SEPRL Institutional Animal Care and Use Committee.
Infection.
Frozen stocks of nalidixic acid-resistant Salmonella serovar Enteritidis (strain SE89-8312) were maintained at −20°C. For each experiment, 3 days prior to infection, Salmonella serovar Enteritidis cells were thawed and cultured onto nutrient agar (Difco/Becton Dickinson Microbiology Systems, Sparks, Maryland) at 37°C for 18 to 24 h. An individual colony of Salmonella serovar Enteritidis was recultured onto nutrient agar and incubated at 37°C for 18 to 24 h. A 1- ml tube of tryptic soy broth (Difco) was then inoculated with isolated colonies from the nutrient agar plate and incubated overnight at 37°C. The Salmonella serovar Enteritidis broth culture was diluted to 10−2 cells in sterile saline, and each bird received a dose of 1 ml per os (9 × 106, 5.6 × 106, and 3 × 106 Salmonella serovar Enteritidis cells in experiments 1, 2, and 3, respectively). At the time of challenge, six food-grade 23-by-28-cm polystyrene trays (Genpack Corp., Glens Falls, NY) were placed on the floor beneath the cages in each room and served as the sites for sampling environmental fecal levels of Salmonella serovar Enteritidis contamination. On testing days, four 1-g fecal samples were obtained from individual trays and placed into separate plastic stomacher bags. The sites were randomly selected each day. The samples were diluted 10-fold in RV broth and then plated onto brilliant green agar containing 20 μg/ml of nalidixic acid and novobiocin (BGNN), using an Autoplate 4000 automatic dilution/plating system (Spiral Biotech, Norwood, MA). After 24 h of incubation at 37°C, counts of Salmonella serovar Enteritidis cells on the plates were made, using a QCount plate reader (Spiral Biotech). For the samples with no detectable growth of Salmonella serovar Enteritidis on the plates, the RV enrichment medium for that sample was plated onto BGNN. These plates were incubated for a further 24 h at 37°C and evaluated for the presence of Salmonella serovar Enteritidis.
Flies and sampling.
Houseflies (Musca domestica) were received as pupae, approximately 2 days prior to emergence as flies, from the USDA/ARS Center for Medical and Veterinary Entomology, Gainesville, FL. Approximately 104 pupae were received in a cardboard container, and the container, with the lid removed, was placed in the bird room. The placement occurred 3 days prior to hen challenge to ensure maximum fly density at the time the birds received the Salmonella serovar Enteritidis. A second batch of 104 pupae was placed in the rooms 7 days later to replenish the fly population. In order to encourage natural visitation by the flies to the birds and the food and feces, no additional food was provided to the flies. On sampling days, one QuikStrike fly abatement strip (Wellmark International, Schaumburg, IL) was placed on a fresh polystyrene tray on the floor of each room. The flies attracted to the bait on the strip consumed a portion of it and rapidly succumbed to the nithiazine toxicant. The dead flies were retrieved, using sterile forceps, one forceps per fly, and deposited into individual zip-sealable plastic bags. The flies were collected individually immediately after knockdown to eliminate cross-contamination after knockdown. Twenty flies per room were collected on each sampling day and transported to the laboratory for culturing. Each bag received 1 ml of RV broth, and the flies were thoroughly crushed and macerated into the broth, using the thumb and forefinger. One hundred microliters of the sample was then spread-plated onto BGNN. The plates and the bags containing the remainder of the fly-RV mixture were incubated for 24 h at 37°C. Following incubation, the BGNN plates were evaluated and Salmonella serovar Enteritidis cell counts were ascertained. For the samples with no detectable Salmonella serovar Enteritidis growth on the plates, the RV enrichment for that sample was plated onto BGNN, and the plates were incubated a further 24 h at 37°C and evaluated for the presence of Salmonella serovar Enteritidis. Samples with no growth on the direct plating but which tested positive in the RV enrichment medium were given an arbitrary count of 9 (1 point below the theoretical detection limit), and samples with no growth in either the direct plating or the RV enrichment medium were given a count of 0.
Localization of Salmonella serovar Enteritidis cells on or in the fly.
Three trials were performed to determine the location of Salmonella serovar Enteritidis cells on or in the fly. In trial 1, seven flies were collected from experiment 2 hens into individual sterile tubes on each of 2 separate days. One milliliter of RV broth was added to each tube, and the tubes were vortexed at maximum speed for 30 s (rinsed). Each fly was then placed into a tube containing 1 ml of bleach (800 ppm chlorine), which was vortexed for 30 s and then placed into a tube containing 1 ml of sterile distilled water and vortexed for a further 30 s. The fly was removed from the water and added to a tube containing 1 ml of RV broth and vortexed for a further 30 s (cleaned). The flies were then transferred individually to 1 ml of fresh RV broth contained in a zip-sealable plastic bag and crushed and macerated between the thumb and forefinger (inside). All samples were incubated at 37°C for 24 h and then plated onto BGNN. Following a further 24-h incubation at 37°C, the plates were examined for the presence of Salmonella serovar Enteritidis. The results from the 2 days were combined, and statistics were run on the combined results. In trial 2, 10 flies were collected from the room containing experiment 3 hens, day 3 postchallenge, and placed into a tube containing 1 ml of phosphate-buffered saline (PBS)-0.5% Tween 20 (Sigma Chemical Co., St. Louis, MO). The tube was vortexed for 30 s, and the PBS-0.5% Tween20 was transferred to 9 ml of RV broth (exterior wash). The fly carcass was then added to 1 ml of bleach (1,000 ppm), vortexed for 30 s, and allowed to sit for 14.5 min. The salivary gland, crop, and gut posterior from the proventriculus from each fly were aseptically excised and placed en mass into 5 ml of RV broth. All samples were incubated for 24 h at 37°C and then plated onto BGNN, and the plates were incubated for a further 24 h at 37°C and examined for the presence of Salmonella serovar Enteritidis. In trial 3, 10 flies were collected from the room containing experiment 3 hens, day 3 postchallenge, and frozen for 24 h. The individual flies were thawed and placed into bleach (1,000 ppm) for 15 min and then dipped into PBS to remove the bleach. The salivary gland, the crop, and then the gut were aseptically excised from each fly and placed into a separate 5-ml RV broth for each tissue. The broths were incubated at 37°C for 24 h and then plated onto BGNN, incubated a further 24 h at 37°C, and examined for the presence of Salmonella serovar Enteritidis.
Transmission of Salmonella serovar Enteritidis via contaminated flies.
Two trials were conducted to examine the transmission of Salmonella serovar Enteritidis to previously unchallenged hens. In trial 1, 200 flies were captured live from experiment 2 hens at day 7 postchallenge and released into a room containing 24 hens from which feed had been removed 3 days previously, a time point chosen because of previous observations of the time of maximal immunosuppression (15). Intestinal shedding of Salmonella serovar Enteritidis by the hens was examined on days 2 and 9 following release of the flies by administering 0.5 ml of 0.5% pilocarpine solution (Sigma) intraperitoneally to each hen and collecting the intestinal secretions released over the next hour onto polystyrene trays placed directly beneath each cage (21). One milliliter of the secretion was added to 9 ml of RV broth. Following 24 h of incubation at 37°C, the broths were plated onto BGNN and were examined for the presence of Salmonella serovar Enteritidis 24 h later. In trial 2, 16 hens (8 hens/room) were transferred to two separate rooms and allowed 7 days to acclimate. Feed was removed for 14 days from the birds in one room, and on day 3 after the removal of feed, the hens in both rooms received five flies per bird, administered with a Pipetteman P1000 (Rainen Instruments, Woburn, MA). The flies, retrieved on day 9 of experiment 3, were loaded five flies per tip into P1000 tips whose ends were clipped to widen the bore diameter and allow passage of the flies. The tips were inserted onto the pipettor, and the flies were expelled down the esophagus of each hen. The hens were sampled for the presence of Salmonella serovar Enteritidis in the crop and intestinal tract. Feed was removed from the fed group of birds the night before the sampling. Intestinal shedding was determined by intraperitoneal pilocarpine administration as described above. Crop colonization was determined as described previously (22). Briefly, 20 cm of tubing (Tygon; interior diameter, 1/8; outer diameter, 3/16; Fisher Scientific) attached to a 10-ml syringe was inserted down the esophagus into the crop, and 5 ml of crop lavage solution (1 M Tris-glycine buffer with 0.25% Tween 20 [pH 7 to 8]) was administered into the crop. The lavage solution, along with the luminal contents of the crop, was aspirated immediately back into the syringe and aseptically transferred into a 15-ml conical tube. Undiluted intestinal and crop samples (100 μl) were spread-plated onto BGNN plates, and 1 ml of each undiluted sample was added to 9 ml of tetrathionate brilliant green broth. The intestinal samples were also diluted 1:10 and plated onto BGNN plates, using an Autoplate 4000 automatic dilution/plating system. The plates and broth enrichments were incubated for 24 h at 37°C, and the plates were examined for growth of Salmonella serovar Enteritidis. For any sample negative for Salmonella serovar Enteritidis, the tetrathionate enrichment broths were plated onto BGNN and examined for the presence of Salmonella serovar Enteritidis cells the next day. Samples with no growth on the direct plating but which were positive in the tetrathionate enrichment broth were given an arbitrary count of 9 (1 point below the theoretical detection limit), and samples with no growth in either the direct plating or the tetrathionate enrichment broth were given a count of 0.
Statistical analyses.
Statistical analyses were performed using GraphPad Instat (GraphPad Software, Inc., San Diego, CA). Unpaired t tests were performed on comparisons between the numbers of Salmonella serovar Enteritidis cells recovered from fly exteriors and interiors in trial 1 and comparisons of chicken crop versus intestinal colonization by the organism following administration of contaminated flies. Analysis of variance with Tukey's multiple comparison test procedures was conducted to compare the numbers of Salmonella serovar Enteritidis cells recovered from the different organs within the fly (trial 3).
RESULTS
Fly contamination.
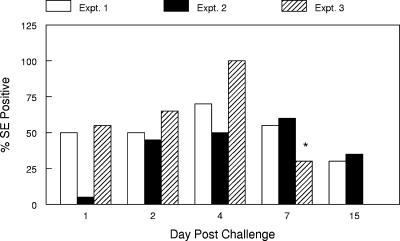
The densities of flies in the rooms at the time the hens were challenged approached 200 flies/m3, which would be considered a low-to-moderate number in commercial egg operations (J. J. Arends, North Carolina State University Department of Entomology, personal communication). Within 48 h, 45 to 50% of the flies in experiments 1 and 2 possessed detectable amounts of Salmonella serovar Enteritidis cells, and recovery of the organisms remained at 50% or greater for the next 5 days (Fig. 1). The numbers of Salmonella serovar Enteritidis cells residing in or on the flies ranged from 9 to 104. In experiment 3, 55% of the flies were positive for Salmonella serovar Enteritidis 24 h postchallenge, and the percentage increased to 100% by day 4 and decreased thereafter (Fig. 1).
FIG. 1.
Recovery of Salmonella serovar Enteritidis (SE) from flies captured in rooms containing infected hens. The results represent the percentage of flies (n = 20 flies/sampling day) that were positive for Salmonella serovar Enteritidis on each sampling day postchallenge in experiments 1, 2, and 3. Expt., experiment; *, flies sampled on day 9 postchallenge in experiment 3.
Fecal contamination with Salmonella serovar Enteritidis.
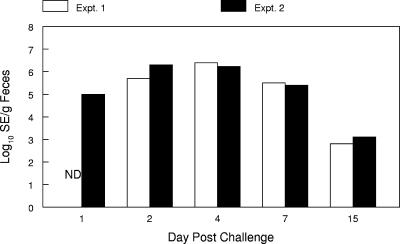
Considerable fecal matter accumulated on the floors beneath the cages over time. Levels of Salmonella serovar Enteritidis ranged from 103 to 107 cells/g feces the first week postchallenge and decreased to 103 to 105 cells/g by the end of the second week in both experiments (Fig. 2).
FIG. 2.
Concentrations of Salmonella serovar Enteritidis (SE) in feces that collected under cages of infected hens over different times postchallenge (n = 4 samples/time period). The results represent the log10 Salmonella serovar Enteritidis cells/gram of feces at the sampling times noted for experiment 1 (Expt. 1) and experiment 2 (Expt. 2). ND, not determined.
Localization of Salmonella serovar Enteritidis on or in the fly.
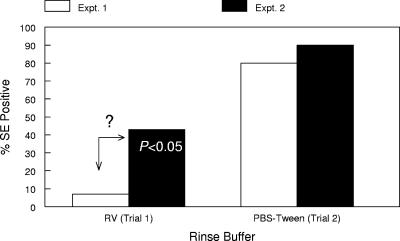
In trial 1, only a small percentage of flies possessed any detectable amount of exterior Salmonella serovar Enteritidis cells, as indicated by culture results from the RV wash of each fly carcass. Conversely, a higher percentage (P < 0.05) of the fly interiors contained the organism (Fig. 3). Repeat experiments using an RV wash or PBS showed similar results. This surprising observation called into question the efficacy of using a simple aqueous wash to break the interaction between the Salmonella serovar Enteritidis cells and the fly. Using a PBS wash solution supplemented with 0.5% Tween 20, a nonionic detergent, resulted in a dramatic increase in the numbers of Salmonella serovar Enteritidis cells recovered from the fly exteriors (Fig. 3), equaling the recovery rate obtained from culturing the fly interior, 80 to 90%. In an effort to determine where within the fly the Salmonella serovar Enteritidis cells resided, salivary glands, crops, and guts were individually cultured; the results are shown in Table 1. No Salmonella serovar Enteritidis cells could be detected in fly salivary glands, and only 10 to 20% of the fly crops had detectable amounts of the organism. Salmonella serovar Enteritidis cells were recovered from all of the fly guts (P < 0.001 versus the numbers for the salivary gland and crop).
FIG. 3.
Simple aqueous rinsing does not remove Salmonella serovar Enteritidis (SE) from fly exteriors. The results represent the percentage of flies that were positive for Salmonella serovar Enteritidis for each sample type in trial 1 (n = 14 flies/sample type) and trial 2 (n = 10 flies/sample type). The number within the bar is the statistical difference between the numbers of Salmonella serovar Enteritidis cells recovered from fly interiors and exteriors. ?, questionable lack of organism.
TABLE 1.
Locations of Salmonella serovar Enteritidis within the digestive tracts of flies retrieved from rooms contaminated with Salmonella serovar Enteritidisa
| Fly no. | Salivary glandb | Cropb | Gutb |
|---|---|---|---|
| 1 | − | − | + |
| 2 | − | − | + |
| 3 | − | − | + |
| 4 | − | − | + |
| 5 | − | − | + |
| 6 | − | − | + |
| 7 | − | − | + |
| 8 | − | − | + |
| 9 | − | − | + |
| 10 | − | + | + |
| No. positive/total | 0/10 | 1/10 | 10/10c |
The results are for individual flies recovered from rooms housing hens challenged with Salmonella serovar Enteritidis on day 3 postchallenge.
The salivary gland, crop, and then the gut were aseptically dissected out from exteriorly disinfected individual flies and cultured. The results represent the presence (+) or absence (−) of Salmonella serovar Enteritidis cells in each tissue from each fly.
P < 0.001 versus results for the crop and salivary gland.
Transmission of S. enteritidis via contaminated flies.
To determine whether flies possessing Salmonella serovar Enteritidis could transmit the organism to naive hens, a study was conducted in which contaminated flies were released into a room containing fasted naive hens. Infection of the hens by Salmonella serovar Enteritidis via exposure to the contaminated flies was not observed (data not shown). To determine whether Salmonella serovar Enteritidis from contaminated flies would be capable of colonizing hens, a second study was conducted in which the birds were administered contaminated flies per os and then monitored for shedding of the organism over the next 3 weeks. An additional parameter, feed withdrawal, was assigned for one group, as this was shown previously to dramatically increase susceptibility to infection (17). As shown in Table 2, minimal colonization of the chicken crop could be observed, while intestinal colonization occurred in approximately 38% of the birds at days 6 (P < 0.05 versus 0% in the crop samples from fed hens) and 13 postchallenge and dropped to 13 to 14% at day 20.
TABLE 2.
Initiation of Salmonella serovar Enteritidis infection in fasted versus nonfasted hens by oral administration of flies contaminated with Salmonella serovar Enteritidisa
| Day postchallenge | % of indicated tissues from fed hens positive for S. serovar Enteritidis
|
P valueb | % of indicated tissues from fasted hens positive for S. serovar Enteritidis
|
P valueb | ||
|---|---|---|---|---|---|---|
| Crop | Intestine | Crop | Intestine | |||
| 6 | 0 | 38 | <0.05 | 13 | 38 | NS |
| 12 | 0 | 25 | NS | 13 | 29 | NS |
| 20 | 0 | 13 | NS | 0 | 14 | NS |
Fed and fasted hens were orally administered flies contaminated with Salmonella serovar Enteritidis, and the presence of the organism in crop and fecal samples was monitored over time.
P values were determined from results for the crop versus those for the intestines. NS, not significant.
DISCUSSION
Flies from environments contaminated with human pathogens readily become contaminated themselves. Rosef and Kapperud showed in 1983 that 51% and 43% of flies captured on chicken farms and piggeries, respectively, in Norway were positive for Campylobacter (31). Bailey et al., were able to isolate Salmonella from 19% of flies captured on broiler farms (2), while Olsen and Hammack (29) found a 22% carrier rate of Salmonella, including Salmonella serovar Enteritidis, in flies captured in facilities housing laying hens. Numerous other studies have reported similar results (10, 27, 37). To the authors' knowledge, no studies have followed the kinetics of colonization with human pathogens of flies exposed to infected animals and their environments. Greenberg et al. (9) fed gnotobiotic houseflies graded doses of S. enterica serovar Typhimurium and found that the flies excreted the organism in their feces at 24 h postchallenge. The percentage of Salmonella excretors was dramatically reduced by 96 h postchallenge, indicating that, for the flies to remain positive for Salmonella over time, they needed a reexposure. Shane et al. (32) showed that houseflies housed within an isolator cabinet containing chickens fecally shedding Campylobacter jejuni became colonized with the organism at 5 days. The current study demonstrated that the common housefly can rapidly become contaminated with Salmonella serovar Enteritidis by residing in an environment housing hens challenged with that organism. This contamination occurred within 24 to 48 h postchallenge of the hens and persisted for at least 2 weeks. Greenberg et al. (9) showed that a reexposure was necessary for Salmonella to persist in the fly host, and a similar reexposure to the contaminated fecal matter was probably necessary to allow the long-term prevalence of Salmonella serovar Enteritidis.
The initial study comparing the frequency of recovery of Salmonella serovar Enteritidis from the fly exterior versus the interior indicated that the organism could be much more readily recovered from the fly interior and that the frequency of recovery from the exterior was very low (Fig. 3). This finding was observed on repeat occasions (data not shown) and is counter to the general consensus regarding flies as vectors of human pathogens via a transfer of the organism through simple contact. Insects possess a waxy cuticle which covers their entire exteriors and serves as a barrier to water loss and also renders them waterproof (26). Adding a lipid solvent such as a detergent dissolves the wax and eliminates the waterproof character of the insect. Indeed, when the detergent Tween 20 was added to the fly rinse buffer in the current study, recovery of Salmonella serovar Enteritidis cells from the fly exterior increased to levels matching that observed for the interior (Fig. 3). These results indicate that the interaction of Salmonella serovar Enteritidis with the fly exterior is more dynamic than simply sitting on the surface of the fly. Further, simple physical contact may not be the primary method of transfer of the organism to different surfaces in a house, and other mechanisms may be more prevalent.
Different microbial pathogens can reside in the fly alimentary tract (4, 7, 9, 12, 32). Greenberg et al. (9) showed that Salmonella serovar Typhimurium fed to flies will reside and multiply in the fly gut. A high percentage of gut samples were culture-positive for Salmonella serovar Typhimurium, while minimal recovery of Salmonella serovar Typhimurium cells from the crop was observed. The results shown in Table 1 of the present study mirror those of Greenberg et al. in that a high percentage, 100%, of the gut samples were positive for Salmonella serovar Enteritidis, while only 15% of the crops had detectable amounts of Salmonella serovar Enteritidis (compared with a previously reported 89% and 11%, respectively, for gut and crop samples [Greenberg et al.] [9]). These results indicate that crop regurgitation by the fly during food collection is probably not a major source of pathogen spread, while defecation by the fly is a much better possibility.
The importance of the carriage of different pathogens by flies lies in their ability to transmit the pathogen to susceptible hosts. In 1985, Shane et al. (32) showed that houseflies taken from isolation cabinets housing Campylobacter-infected chickens shedding the bacterium fecally and introduced into a second isolation cabinet containing naive chickens could transmit Campylobacter to these recipient birds. In the current study, transmission of Salmonella serovar Enteritidis was attempted through the introduction of flies collected from rooms containing infected hens into a room containing naive hens. To increase the potential for transmission to occur, feed was withdrawn from the recipients, mimicking a layer industry management tool, induced molting, used to achieve multiple egg-laying cycles from aging flocks. Prior to January 2006, withdrawal of feed until the flock dropped 30% in body weight, 10 to 14 days, was the primary method for flock recycling. This methodology has since been replaced in the U.S. with less-stressful diet regimens (35), while in Latin America and the Far East, responsible for more than 50% of egg production worldwide, feed withdrawal remains the primary method for molting flocks. Earlier studies have shown that feed withdrawal depresses cellular immunity in the birds (15, 16), resulting in an increased severity of infection by Salmonella serovar Enteritidis (17, 18, 19, 20, 21) and dramatically increasing the susceptibility of hens to infection (17). In the present study, this increased susceptibility had no apparent impact on the transmission of Salmonella serovar Enteritidis to the recipient birds, as no birds were found to be colonized by the organism. There are several possible explanations for the discrepancy. Campylobacter and S. enteritidis are entirely different organisms, and their transmission capabilities may therefore differ. Also, while birds in both studies received equivalent numbers of flies, the birds in the earlier study were housed in a relatively small, contained space, a Horsfall isolation cabinet (0.6 m by 0.6 m by 0.6 m), while the hens in the present study were housed in a 5 m by 3 m by 3 m animal room. The latter situation dramatically expanded the space that the flies could traverse, eliminated the intimate interaction brought on by small, enclosed spaces, and decreased the fly density from 1,000 flies/m3 to 5 flies/m3. However, Salmonella serovar Enteritidis-contaminated flies can transmit the organism when they are fed to naive hens (Table 2), indicating that transmission of Salmonella serovar Enteritidis by introducing contaminated flies into a room containing naive hens may be possible if a larger number of contaminated flies were used in the study or, conversely, by housing the recipient birds in isolation cabinets to enhance the contact between fly and bird. Note that the number of flies released in this study was relatively low and represented a density substantially below that commonly observed in commercial flocks.
The chicken crop is an enlargement or outpouching of the esophagus just proximal to the proventriculus, or glandular stomach. The primary function of the crop is food storage while the stomach is full. The chicken crop was shown to routinely harbor Salmonella and was touted as an important source of broiler carcass contamination during processing (3, 5, 11). Birds challenged with Salmonella serovar Enteritidis showed equal levels of colonization of the crop and intestinal tract (23). Interestingly, few of the crops from the recipient hens were culture positive for Salmonella serovar Enteritidis. Why these birds differed from those in previous studies remains to be determined. It may simply be a function of bacterial density, in that most challenge studies use 104- to 106-cell challenge doses, while the flies in the current study generally carried many fewer organisms (range, 9 to 104 organisms). Further, the crop tissues were bathed in the organism following oral challenge, while the fly-derived Salmonella would be trapped in and on the fly and just transit on through the organ. In the field broiler (5, 11) studies, the birds spent their life spans on contaminated poultry litter and, as a certain degree of coprophagy occurs during broiler grow-out, the crop was regularly recontaminated and recolonized.
The present study reinforces what has been known for a long time, that flies residing in a contaminated environment will, themselves, become contaminated. However, to the authors' knowledge, the rapidity of contamination of the flies and the localization of the organism within the individual insects following exposure to a contaminated environment has not been previously demonstrated. Transmission via the fly vector appears to be a somewhat more complex situation, requiring more than just the fly walking across a counter or landing on exposed foodstuffs. As contaminated flies administered to hens will initiate an infection, residing on or in a fly does not eliminate the infectivity of the Salmonella serovar Enteritidis organism. Although we did not observe direct transmission by releasing infected flies into a room with healthy birds, the number of flies used in the challenge was modest and probably below the threshold levels necessary to result in effective transmission of Salmonella serovar Enteritidis from flies to poultry. Additional work is needed with higher fly challenge densities and to determine the relative roles of fly ingestion and contamination of foods by fly fecal and vomit deposits. The mechanics and parameters of pathogen spread by flies still require a more in-depth analysis before this “simple” phenomenon is understood.
Acknowledgments
We thank Lara Vaughn and Joyce Jacks for their excellent technical assistance during the performance of the studies. Thanks also to Lisa Durso, USDA/ARS MARC, Clay Center, NE, for the suggestion of including detergent in the fly rinse to release organisms adhering to the surface of the fly. A special thanks is extended to J. J. Arends, Department of Entomology, North Carolina State University, Raleigh, NC, for sharing his expertise on fly levels found in commercial layer operations.
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Ash, N., and B. Greenberg. 1980. Vector potential of the German cockroach (Dictyoptera: Blattellidae) in dissemination of Salmonella enteritidis serotype typhimurium. J. Med. Entomol. 17:417-423. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, J. S., N. J. Stern, P. Fedorka-Cray, S. E. Craven, N. A. Cox, D. E. Cosby, S. Ladely, and M. T. Musgrove. 2001. Sources and movement of Salmonella through integrated poultry operations: a multistate epidemiological investigation. J. Food Prot. 64:1690-1697. [DOI] [PubMed] [Google Scholar]
- 3.Barrow, P. A., J. M. Simpson, and M. A. Lovell. 1988. Intestinal colonisation in the chicken by food-poisoning Salmonella serotypes; microbial characteristics associated with faecal excretion. Avian Pathol. 17:571-588. [DOI] [PubMed] [Google Scholar]
- 4.Calibeo-Hayes, D., S. S. Denning, S. M. Stringham, J. S. Guy, L. G. Smith, and D. W. Watson. 2003. Mechanical transmission of turkey coronavirus by domestic houseflies (Musca domestica Linnaeaus). Avian Dis. 47:149-153. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, J. R., J.-R. Bisaillon, Y. Labbe, C. Poppe, and C. F. Langford. 1998. Salmonella prevalence in crops of Ontario and Quebec broiler chickens at slaughter. Poult. Sci. 77:1497-1501. [DOI] [PubMed] [Google Scholar]
- 6.Cogan, T. A., and T. J. Humphrey. 2003. The rise and fall of Salmonella Enteritidis in the UK. J. Appl. Microbiol. 94:114S-119S. [DOI] [PubMed] [Google Scholar]
- 7.De Jesús, A. J., A. R. Olsen, J. R. Bryce, and R. C. Whiting. 2004. Quantitative contamination and transfer of Escherichia coli from foods by houseflies, Musca domestica L. (Diptera: Muscidae). Int. J. Food Microbiol. 93:259-262. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg, B. 1971. Flies and disease. In Ecology, classification and biotic associations, vol. 1. Princeton University Press, Princeton, NJ.
- 9.Greenberg, B., J. A. Kowalski, and M. J. Klowden. 1970. Factors affecting the transmission of Salmonella by flies: natural resistance to colonization and bacterial interference. Infect. Immun. 6:800-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hald, B., H. Skovgärd, D. D. Bang, K. Pedersen, J. Dybdahl, J. B. Jespersen, and M. Madsen. 2004. Flies and Campylobacter infection of broiler flocks. Emerg. Infect. Dis. 10:1490-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargis, B. M., D. J. Caldwell, R. L. Brewer, D. E. Corrier, and J. R. DeLoach. 1995. Evaluation of the chicken crop as a source of Salmonella contamination for broiler carcasses. Poult. Sci. 74:1548-1552. [DOI] [PubMed] [Google Scholar]
- 12.Hawley, J. E., L. R. Penner, S. E. Wedberg, and W. Kulp. 1951. The role of the house fly, Musca domestica, in the multiplication of certain enteric bacteria. Am. J. Trop. Med. Hyg. 31:572-582. [DOI] [PubMed] [Google Scholar]
- 13.Henzler, D. J., D. C. Kradel, and W. M. Sischo. 1998. Management and environmental risk factors for Salmonella enteritidis contamination of eggs. Am. J. Vet. Res. 59:824-829. [PubMed] [Google Scholar]
- 14.Henzler, D. J., and H. M. Opitz. 1992. The role of mice in the epizootiology of Salmonella enteritidis infection on chicken layer farms. Avian Dis. 36:625-631. [PubMed] [Google Scholar]
- 15.Holt, P. S. 1992. Effects of induced moulting on immune responses of hens. Br. Poult. Sci. 33:165-175. [DOI] [PubMed] [Google Scholar]
- 16.Holt, P. S. 1992. Effect of induced molting on B cell and CT4 and CT8 T cell numbers in spleens and peripheral blood of white leghorn hens. Poult. Sci. 71:2027-2034. [DOI] [PubMed] [Google Scholar]
- 17.Holt, P. S. 1993. Effect of induced molting on the susceptibility of white leghorn hens to a Salmonella enteritidis infection. Avian Dis. 37:412-417. [PubMed] [Google Scholar]
- 18.Holt, P. S. 1995. Horizontal transmission of Salmonella enteritidis in molted and unmolted laying chickens. Avian Dis. 39:239-249. [PubMed] [Google Scholar]
- 19.Holt, P. S., R. J. Buhr, D. L. Cunningham, and R. E. Porter, Jr. 1994. Effect of two different molting procedures on a Salmonella enteritidis infection. Poult. Sci. 73:1267-1275. [DOI] [PubMed] [Google Scholar]
- 20.Holt, P. S., N. P. Macri, and R. E. Porter, Jr. 1995. Microbiological analysis of the early Salmonella enteritidis infection in molted and unmolted hens. Avian Dis. 39:55-63. [PubMed] [Google Scholar]
- 21.Holt, P. S., and R. E. Porter, Jr. 1992. Microbiological and histopathological effects of an induced molt fasting procedure on a Salmonella enteritidis infection in chickens. Avian Dis. 36:610-618. [PubMed] [Google Scholar]
- 22.Holt, P. S., L. E. Vaughn, R. K. Gast, and H. D. Stone. 2002. Development of a lavage procedure to collect crop secretions from live chickens for studying crop immunity. Avian Pathol. 31:589-592. [DOI] [PubMed] [Google Scholar]
- 23.Holt, P. S., L. E. Vaughn, R. W. Moore, and R. K. Gast. 2006. Comparison of Salmonella enterica serovar enteritidis levels in crops of fed or fasted infected hens. Avian Dis. 50:425-429. [DOI] [PubMed] [Google Scholar]
- 24.Kopanic, R. J., B. W. Sheldon, and C. G. Wright. 1994. Cockroaches as vectors of Salmonella: laboratory and field trials. J. Food Prot. 57:125-132. [DOI] [PubMed] [Google Scholar]
- 25.Krabisch, V. P., and P. Dorn. 1980. The importance of living vectors for the dissemination of Salmonellae in broiler flocks. Berl. Munch. Tierarztl. Wschr. 93:232-235. [PubMed] [Google Scholar]
- 26.Machin, J. 1980. Cuticle water relations: towards a new cuticle water-proofing model, p. 79-103. In M. Locke and D. S. Smith (ed.), Insect biology in the future. Academic Press, New York, NY.
- 27.Mian, L. S., H. Maag, and J. V. Tacal. 2002. Isolation of Salmonella from muscoid flies at commercial animal establishments in San Bernardino County, California. J. Vector Ecol. 27:82-85. [PubMed] [Google Scholar]
- 28.Olsen, A. R. 1998. Regulatory action for filth and other extraneous material. III. Review of flies and foodborne enteric disease. Reg. Toxicol. Pharmacol. 28:199-211. [DOI] [PubMed] [Google Scholar]
- 29.Olsen, A. R., and T. S. Hammack. 2000. Isolation of Salmonella spp. from the housefly, Musca domestica L., and the dump fly, Hydrotaea aenescens (Wiedemann) (Diptera: Muscidae), at caged-layer houses. J. Food Prot. 63:958-960. [DOI] [PubMed] [Google Scholar]
- 30.Patrick, M. E., P. M. Adcock, T. M. Gomez, S. F. Altekruse, B. H. Holland, R. V. Tauxe, and D. L. Swerdlow. 2004. Salmonella enteritidis infections, United States, 1985-1999. Emerg. Infect. Dis. 10:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosef, O., and G. Kapperud. 1983. House flies (Musca domestica) as possible vectors of Campylobacter fetus subsp. jejuni. Appl. Environ. Microbiol. 45:381-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shane, S. M., M. S. Montrose, and K. S. Harrington. 1985. Transmission of Campylobacter jejuni by the house fly (Musca domestica). Avian Dis. 29:384-391. [PubMed] [Google Scholar]
- 33.Skov, M. N., A. G. Spencer, B. Hald, L. Petersen, B. Nauerby, B. Carstensen, and M. Madsen. 2004. The role of litter beetles as potential reservoir for Salmonella enterica and thermophilic Campylobacter spp. between broiler flocks. Avian Dis. 48:9-18. [DOI] [PubMed] [Google Scholar]
- 34.Tauxe, R. V. 2001. Salmonella: a postmodern pathogen. J. Food Prot. 54:563-568. [DOI] [PubMed] [Google Scholar]
- 35.United Egg Producers. 2006. Animal husbandry guidelines for U.S. egg laying flocks. http://www.uepcertified.com/docs/2006_UEPanimal_welfare_guidelines.pdf.
- 36.Uyttendaele, M. R., J. M. Debevere, R. M. Lips, and K. D. Neyts. 1998. Prevalence of Salmonella in poultry carcasses and their products in Belgium. Int. J. Food Microbiol. 40:1-8. [DOI] [PubMed] [Google Scholar]
- 37.Wales, A., M. Breslin, and R. Davies. 2006. Assessment of cleaning and disinfection in Salmonella-contaminated poultry layer houses using qualitative and semi-quantitative culture techniques. Vet. Microbiol. 116:283-293. [DOI] [PubMed] [Google Scholar]