Abstract
PURPOSE
To introduce a nondestructive technique for characterization of corneal stiffness, determine measurement precision, and investigate comparative stiffness values along central, radial, and circumferential vectors in porcine corneas. The effects of epithelial debridement, relaxing incisions, and crosslink-mediated stiffening on surface wave velocity are also studied.
METHODS
A handheld prototype system was used to measure ultrasound surface wave propagation time between two fixed-distance transducers along a ten-position map. Repeatability was assessed with replicate measurements in 6 porcine corneas. In 12 porcine globes with controlled intraocular pressure (IOP), serial measurements were performed before and after epithelial removal, then after 250- and 750-μm-deep relaxing incisions. In human globes with constant intravitreal pressure, central wave velocity and transcorneal IOP measurements were compared before and after collagen cross-linking.
RESULTS
Measurement repeatability across all regions was between 2.2% and 8.1%. Epithelial removal resulted in increases in measured stiffness in 67% of eyes, but statistical power was insufficient to detect a systematic change. Wave velocity across a central incision decreased significantly after 250-μm keratotomy (P<.001), but did not undergo a significant further decrease with deeper keratotomy. Meridional stiffness changes consistent with coupling effects were detected after keratotomy. Surface wave velocity and transcorneal IOP measurements increased markedly after collagen cross-linking despite maintenance of a constant IOP.
CONCLUSIONS
Handheld corneal elastometry provides a repeatable measure of regional stiffness changes after relaxing incisions and collagen cross-linking in in vitro experiments. Surface wave elastometry allows focal assessment of corneal biomechanical properties that are relevant in refractive surgery, ectatic disease, and glaucoma.
The cornea functions as a mechanical barrier to injury and a transparent scaffold for the eye's primary refracting surface, the tear film. The material properties of the cornea are integral to each of these functions and impact its response to disease and surgery. To better ascertain the relevance of these properties in clinical ophthalmology, techniques that are capable of characterizing material behavior in a nondestructive fashion are needed. Potential applications include patient-specific biomechanical optimization of surgical algorithms in corneal and keratorefractive surgery, early clinical detection of corneal ectatic disease,1,2 improved accuracy of intraocular pressure (IOP) measurement,3,4 quantitative assessment of corneal wound healing,5-7 and evaluation of the biomechanical performance of tissue-engineered corneas.8
Specific mechanisms of biomechanical shape change in photoablative procedures such as phototherapeutic keratectomy (PTK), photorefractive keratectomy (PRK), and LASIK have been described.9,10 Corneal stiffness is likely to be a critical modulator of these responses,11,12 and techniques for quantifying key elements of the corneal biomechanical phenotype prior to keratorefractive surgery would be of considerable use in screening surgical candidates and planning subsequent surgery. Computational models using finite element analysis and other analytical methods have been used to model the structural effects of refractive surgery11,13-18 and to estimate the safe limits of surgical ablation for preventing iatrogenic ectasia.2,12 Similar models have assessed the role of corneal rigidity in applanation tonometry.19,20 The ability of these numerical models to predict actual corneal behavior relies on appropriate geometric modeling and, moreover, assignment of valid material properties to model elements. Although marked clinical advances have been made in three-dimensional corneal imaging, in vivo measurement of biomechanical properties such as the elastic modulus—a representation of the stiffness of a material—remains a challenge. Furthermore, estimates of the corneal elastic modulus obtained from the experimental literature differ by orders of magnitude.21 This variability probably exceeds any plausible biological variability and instead reflects the challenges of obtaining representative data from ex vivo experiments with wide-ranging experimental conditions. Because the sensitivity of numerical model solutions to this error is high,12 approximations culled from the literature are inadequate when the goal is to generate custom simulations for optimizing surgical dosing or modeling ectasia risk in individual patients.
In the present study, we investigate a technique for characterizing corneal stiffness using surface wave propagation velocity. The velocity of an acoustic surface wave traveling parallel to the orientation of collagen fibers in a viscoelastic material is a function of its stiffness.22 In the current study, experiments were designed first to assess normative values and regional repeatability of surface wave velocities measured with a prototype wave propagation elastometer in porcine globes. Additional experiments specifically investigated any confounding effect of the corneal epithelium on measurement of corneal stiffness, which is primarily a function of the underlying collagenous components, and estimated the effective sampling depth of elastometer measurements. Finally, keratotomy and collagen cross-linking techniques were used to study changes in corneal surface wave velocity from interventions known to either decrease or increase corneal stiffness, respectively.
MATERIALS AND METHODS
Surface Wave Elastometer System
A clinical prototype system was used to measure sonic wave propagation time between two transducers positioned on the corneal surface (Sonic Eye; PriaVision Inc, Menlo Park, Calif). A handheld probe housed two piezoelectric transducers, one a transmitter and the other a receiver, with a fixed separation of 4.5 mm (Fig 1). The inter-transducer distance was selected to allow sampling of a substantial arc length of cornea, to minimize nonlinear behavior due to near-field effects,23,24 and to provide sufficient path length for development of a Rayleigh wave whose velocity might ultimately be related mathematically to the elastic (Young's) modulus.23
Figure 1.
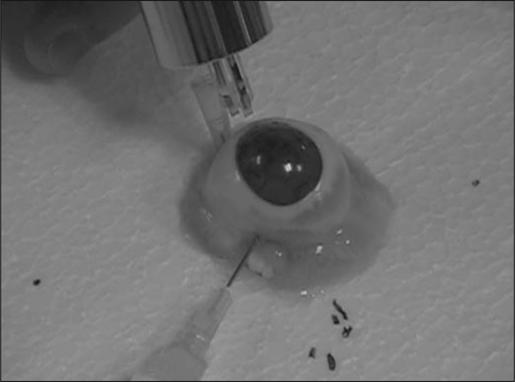
The elastometer probe incorporates surface-contact transducers and measures the time of flight of a sonic wave at the corneal surface.
The transducers were mounted on a recoiling support to minimize the risk of epithelial trauma. The emitting contact was triggered via a footswitch to produce a 4- to 5-kHz oscillatory force with an acoustic energy less than 10 microwatts and duration of approximately 1 millisecond. The time required for the transmitted wave to reach threshold amplitude at the receiving probe was measured and recorded via a USB connection to a laptop computer. Replicate measurements could be obtained at a rate of 1 per second with continuous pressure on a triggering foot pedal. A software interface allowed conversion of time-of-flight data to a velocity display in meters per second (m/s), and velocity data were exported to Excel (v. 11, SP2; Microsoft Corp, Redmond, Wash) and Minitab (v. 14.20; Minitab Inc, State College, Pa) for statistical analysis.
A measurement map was devised to combine potentially informative regional and directional elastometer measurements in a single template that could be applied to current and future studies (Fig 2). The location and orientation of each measurement position was selected to increase sensitivity for detecting biomechanical differences between different corneal meridia,25 between the corneal center and periphery,26 and to account for potential differences due to regional variations in the predominant collagen fibril direction27 and extent of collagen interweaving.28 Peripheral measurements were obtained with the transducer tips just inside the limbus to provide a repeatable visual landmark for measurement. A standardized elastomer was measured in replicate (n=10) prior to each experiment to allow future correlation of surface wave velocity measurements to a known elastic modulus.
Figure 2.
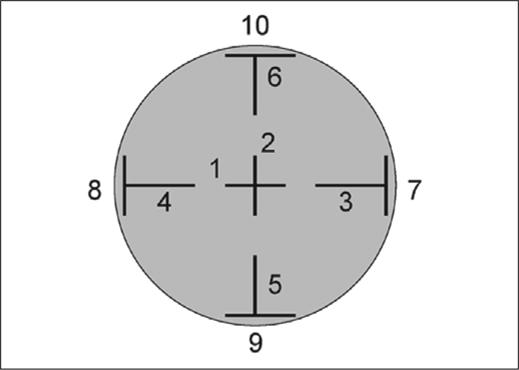
Measurement template incorporating 10 regional and directional measurements of surface wave velocity (central [1, 2], radial peripheral [3−6], and circumferential peripheral [7−10]). Black bars demarcate the span of the transducer tips at each location (4.5 mm), and numbers indicate the order in which each measurement was obtained.
Repeatability of Surface Wave Velocity Measurements
Repeatability in a porcine whole-globe model was expressed as the coefficient of variation (COV), which is the ratio of the standard deviation to the mean expressed as a percentage. In this study subset, regional velocities were analyzed solely for the purposes of calculating repeatability and IOP was not controlled. In subsequent experiments where the objectives included determining relative values for each region, IOP was explicitly controlled because of its influence on wave velocity measurements (Dadhania and Dupps, unpublished data, 2005).
Six fresh porcine globes with intact epithelia (VisionTech Inc, Mesquite, Tex) were mounted individually on a custom Styrofoam holder. To assess both intra- and inter-session repeatability, three sets of 10 serial measurements were obtained in the elastomer and then at each position on the porcine corneas (see Fig 2). Each measurement within a series of 10 replicates was performed at a rate of 1 per second without lifting the probe, then the probe was lifted and replaced between each set of 10. Intra-session repeatability was obtained by calculating the average COV within each set of 10 replicates. Inter-session repeatability was estimated from the standard deviation of the means from the 3 sets of 10 expressed as a COV. Because the sidedness and thus the orientation of the porcine globes were unknown, regional repeatability results were aggregated into orientation-independent categories where COV was averaged for the following regions in Figure 2: central (positions 1 and 2), radial (positions 3−6), and circumferential (positions 7−10).
Directional/Regional Stiffness, Effects of Epithelial Removal, and Effects of Relaxing Incisions
The next series of experiments was designed to 1) characterize any regional variation in stiffness in porcine eyes, 2) quantify any confounding effects of the corneal epithelium on stromal wave velocity by comparing measurements obtained before and after epithelial debridement, 3) determine the effect of central keratotomy depth on velocity, and 4) in a related analysis, to estimate the effective sampling depth of the prototype device in porcine corneas.
Twelve additional fresh porcine eyes were secured in a supine position in a custom Styrofoam eye holder. The vitreous chamber was cannulated with a 23-gauge needle (BD, Franklin Lakes, NJ) attached both to a fluid column of 0.9% normal saline and a transducer (Biotrans 2; Biosensors International Inc, Singapore) fixed at the level of the globe. Intraocular pressure was monitored on a continuous-display digital pressure monitor (Infinity SC9000XL; Drager Medical, Lubeck, Germany). After calibration and zeroing were performed, infusion pressure was controlled with a squeeze valve to maintain an IOP of 15 mmHg throughout the experiment. Serial measurements at all 10 positions were performed on intact, dry epithelium and then repeated after epithelial removal with a 0064 Beaver blade (BD). Because the epithelium is a cellular layer devoid of tension-bearing collagenous lamellae and because the stroma is the target of interest, we wished to rule out the possibility of 1) generation of an entirely intraepithelial propagation wave that never samples the stroma or 2) significant epithelial dampening of the stromal signal. Next, a diamond keratotomy knife (DGH KOI Inc, Shermans Dale, Pa) was used to create a 250-μm deep, 3-mm long vertical incision in the central stroma (along position 2 in Fig 2). After another full set of elastometer measurements was obtained, the same incision was then deepened to 750 μm and the measurements were repeated. At each corneal position and in the elastomer, 10 replicate measurements were obtained without lifting the probe. The average of the last 5 measurements was extracted from the database for analyses of the COV and wave velocity changes due to each intervention. We used the average of the last 5 measurements after observing that these measurements were generally more stable than the first 5. Velocity differences at each step were assessed for significance by paired Student t tests at the P<.05 level. Power calculations to evaluate the statistical sensitivity to detect a change in corneal properties after epithelial removal and after 750-μm keratotomy were performed retrospectively using the public domain software package R (v. 2.3.1; R Foundation for Statistical Computing, http://www.r-project.org) for two-sided comparisons of paired samples with α=.05.
Effect of Crosslink-Mediated Stiffening on Wave Velocity and Corneal IOP Measurement
In a final experiment assessing the effect of collagen stiffening on surface wave velocity and transcorneal IOP measurement, two unpaired human research globes were obtained from the Cleveland Eye Bank (age 67 and 88 years, 7 and 28 days after preservation for eye 1 and 2, respectively). Eyes were secured in the mount, de-epithelialized, and maintained at an intravitreal pressure of 30 mmHg by the technique described above. Central corneal thickness (CorneaGage Plus; Sonogage, Mentor, Ohio) was measured before and after cross-linking. Vertical and horizontal wave velocity measurements were averaged in each eye before and after stromal collagen cross-linking, as were triplicate IOP measurements obtained with a calibrated Tono-Pen XL (Medtronic Solan, Jacksonville, Fla) and pneumatonometer (Mentor O&O Inc, Norwell, Mass). Collagen stiffening was achieved with a 45-minute immersion in 4% glutaraldehyde diluted from 8% electron microscopy grade stock (Polysciences, Warrington, Pa) in phosphate buffered saline (Sigma, St Louis, Mo), similar to previously described techniques.29,30
RESULTS
Repeatability of Surface Wave Velocity Measurements
The repeatability of surface wave velocity measurements in tissue is summarized in Table 1 for 6 porcine corneas with intact epithelium. The synthetic elastomer material provided with the probe was measured at the beginning of each 10-position tissue measurement sequence, which resulted in a total of 53 independent measurements with a mean wave velocity of 121.8±5.8 m/s. The average intra-session COV for the elastomer was 1.1±0.7%.
TABLE 1.
Coefficient of Variation of Surface Wave Velocity Measurements in Epithelialized Porcine Corneas (n=6)
|
Coefficient of Variation (%) |
||
|---|---|---|
| Region (Position number) | Intra-Measurement | Inter-Measurement |
| Central (1,2) | 2.5±0.1 | 8.2±0.3 |
| Radial (3,4,5,6) | 2.5±0.4 | 8.9±2.4 |
| Circumferential (7,8,9,10) | 1.9±0.4 | 7.3±1.7 |
| Overall | 2.2±0.3 | 8.1±1.7 |
Effect of Epithelial Debridement on Surface Wave Velocity
A comparison of mean regional velocities in 12 pressurized porcine corneas before and after epithelial debridement is shown in Table 2. Mean central velocity (aggregate of positions 1 and 2) was 110±39 m/s with intact epithelium and 128±20 m/s after epithelial removal. Paired Student t tests revealed no significant differences between regions (P>.05) within the pre-debridement and post-debridement sessions. As illustrated in Table 2, no systematic increases or decreases in measured stiffness were detected after epithelial removal at any of the aggregate positions (P>.05). However, a retrospective power calculation based on the mean difference and actual variance revealed that the statistical power to detect such a difference was only 47%. This was most likely attributable to the variable epithelial status of the postmortem porcine eyes, which contributed to the large standard deviation noted in Table 2 in pre-debridement eyes. Removal of the epithelium did, however, increase the measured stiffness in 67% of corneas and reduce inter-subject differences in velocity measurements based on a reduction in the inter-subject standard deviation (Table 2).
TABLE 2.
Regional Analysis of Wave Velocity in 12 Porcine Corneas with IOP of 15 mmHg Subjected to Epithelial Debridement and Central Vertical Keratotomy
| Region | Velocity With Epithelium (m/s) | Velocity Without Epithelium (m/s) | P Value | Velocity After 250-μm Incision (m/s) | P Value | Velocity After 750-μm Incision (m/s) | P Value |
|---|---|---|---|---|---|---|---|
| Central (1,2) | 110±39 | 128±20 | .12 | 106±19 | <.001 | 100±22 | .22 |
| Across incision (1) | 108±42 | 130±28 | .11 | 75±18 | <.001 | 62±26 | .10 |
| Along incision (2) | 112±38 | 127±20 | .18 | 137±26 | .11 | 138±28 | .79 |
| Radial (3,4,5,6) | 120±29 | 120±17 | .98 | 124±18 | .36 | 134±22 | .02 |
| Perpendicular to incision (3,4) | 111±30 | 121±20 | .20 | 115±19 | .34 | 128±26 | .048 |
| Parallel to incision (5,6) | 129±33 | 118±22 | .23 | 133±21 | .03 | 140±23 | .24 |
| Circumferential (7,8,9,10) | 122±38 | 124±23 | .70 | 125±26 | .76 | 121±21 | .35 |
Note. Represented as mean±standard deviation, paired t test for comparison to immediately preceding step, significant differences in bold.
Effect of Serial 250-μm and 750-μm Incisions on Surface Wave Velocity
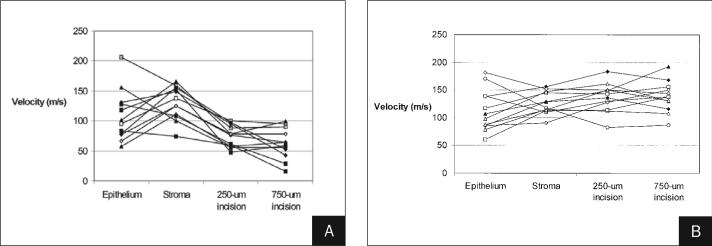
A significant decrease in wave velocity occurred across the site of a vertical 250-μm keratotomy incision and is summarized in Table 2. Extension of the wound depth to 750 μm did not produce a significant additional reduction in cross-incisional wave velocity (P=.10), with a power of 46% to detect the observed decrease at the α=.05 level. An initial trend toward increased wave velocity in the central vertical measurement parallel to and overlying the incision was not significant (P=.11 at 250 μm, P=.79 at 750 μm, Table 2), with a similarly low relative statistical power. A comparison of paracentral radial velocities in the horizontal meridian (positions 3 and 4, along fibers that cross the plane of the incision) and the vertical meridian (positions 5 and 6, along fibers that are not disrupted by the incision) revealed no significant difference prior to incision (121±20 vs 118±22 m/s, respectively, P=.30). After 250-μm vertical keratotomy, however, the difference between horizontal (115±19 m/s) and vertical meridians (133±21 m/s) was significant (P=.03). This was accompanied by a significant increase in vertical radial stiffness (from 118±22 to 133±21 m/s, P=.03) with an insignificant trend toward loss of stiffness in the peripheral cross-incisional meridian (P>.05, Table 2). A trend toward additional vertical meridian stiffening with deeper keratotomy was not significant (P>.05, Table 2). These results suggest a stiffening of the un-disrupted in-plane collagen fibers, with or without relaxation of the peripheral fibers crossing the incision plane with more superficial incisions. Radial stiffness in the entire aggregate paracentral region (positions 3, 4, 5, and 6) increased significantly when central incisions were deepened to 750 μm (P=.02), which was accompanied by an unexpected increase in radial stiffness perpendicular to the incision (in the direction of cut fibers) with the deepest keratotomies (P=.048, Table 2). Figure 3 illustrates the sequential changes in wave velocity at central positions 1 and 2 for all 12 eyes over all 4 measurement steps.
Figure 3.
Sequential results of central stiffness measurements at position A) 1 and B) 2 in 12 porcine corneas maintained at IOP 15 mmHg. Each cornea was measured before debridement, after debridement, after a 3-mm long, 250-μm deep central keratotomy was performed along position 2 (see Figure 2), and after extension of the incision to 750 μm.
Effect of Collagen Cross-linking on Surface Wave Velocity and Transcorneal IOP Measurement
Corneal cross-linking markedly increased both the surface wave velocity and transcorneal IOP by pneumatonometer and Tonopen while direct IOP was carefully maintained at 30 mmHg (Table 3). Central corneal thickness decreased slightly in both eyes by ultrasound pachymetry during the experiment (from 934 μm to 884 μm in eye 1 and from 842 μm to 763 μm in eye 2). At the completion of the experiment, trephination of the second globe to the point of anterior chamber entry reduced the intravitreal pressure to 6 mmHg while transcorneal IOP remained 64 mmHg by pneumatonometer and 86 mmHg by Tonopen.
TABLE 3.
Effect of Collagen Crosslinking on Surface Wave Velocity and Transcorneal Intraocular Pressure (IOP) With Intravitreal IOP Maintained at 30 mmHg
|
Before Cross-linking |
After Cross-linking With 4% Glutaraldehyde |
|||||
|---|---|---|---|---|---|---|
| Eye | Pneumo (mmHg) | Tonopen (mmHg) | Velocity (m/s) | Pneumo (mmHg) | Tonopen (mmHg) | Velocity (m/s) |
| 1 | 33.5 | 35 | 80±3 | 71 | 87 | 145±5 |
| 2 | 31 | 36 | 79±4 | 79.5 | 89 | 147±5 |
DISCUSSION
We describe a handheld ultrasonic tool for non-destructive measurement of sonic wave velocity, an analog of corneal stiffness. Other investigators, including Wang et al31 and Liu and Roberts,32 also explored the potential role of ultrasound propagation measurements for characterizing corneal elasticity. Sonic wave velocimetry has been described in the dermatologic literature24,33-36 and has been used to measure age-related increases in tissue stiffness,33,37 softening effects of tissue hydration and skin creams,24 and sclerosing effects of fractionated radiation for post-lumpectomy breast tumors on breast skin.38 When acoustic waves in the 0.5 to 30 kHz frequency range are used, propagation speed in skin is related to the density and stiffness of skin.35 Analyses of corneal signals obtained with our prototype elastometer have demonstrated an oscillatory frequency within this range (4 to 5 kHz). Using a similar wave propagation technique with a 1.5-mm transducer separation and an oscillation frequency of 5.7 kHz, Vexler et al24 reported shear wave propagation speeds in human volar forearm skin ranging from 40 to 70 m/s. The central corneal velocities obtained in the present experiments—approximately 80 m/s in the edematous human eyes and 120 m/s in porcine cornea—were slightly higher but comparable in magnitude.
In porcine tissue, central, radial, and circumferential measurements obtained with the elastometer were repeatable using a clinically feasible free-handed approach. Tests on the standard elastomer provided a repeatability estimate in an idealized measurement substrate and produced a COV of only 1.1%. In tissue, the COV was expectedly slightly higher. Intra-session repeatability, which incorporated short-term measurement drift due to tissue factors such as viscoelastic creep, stress relaxation, and IOP change from probe pressure, was 2.2% on average. The inter-session repeatability, which estimated the error associated with probe repositioning and associated differences in indentation pressure, was slightly higher (8.1%) and, like the former, did not vary by region. The confidence of our estimate of inter-session repeatability could be adversely affected by the small number (n=3) of comparative sessions, but it provided a practical estimate of measurement repeatability that justified proceeding with the subsequent experiments. These metrics do not account for differences that can be expected in living eyes from diurnal variation, variable consistency of the tear film, and other factors that are more readily controlled in a laboratory setting.
Although a statistically significant alteration of corneal stiffness measurements across the cohort could not be detected with epithelial debridement (Table 2), measurable differences were noted in individual eyes (see Fig 3). In measurements of the central cornea, an insignificant trend toward increased stiffness was observed after epithelial removal (Table 2). The power to detect a difference in central measurements before and after epithelial removal was low (47%); however, Figure 3 more aptly illustrates the potential for epithelium to affect corneal stiffness measurements in individual eyes, where central wave velocity increased after debridement in 67% (8/12) of eyes. Also of note, eyes with the highest stiffness measurements atop epithelium accounted for 3 of the 4 eyes in which stiffness decreased after debridement. Because epithelial debridement reduced measurement variability between porcine corneas (according to the standard deviations in Table 2), we recommend debridement for maximizing statistical power in laboratory investigations when appropriate. Whereas the integrity of the epithelium is relatively inconsistent in postmortem tissue models, we might expect less variance in in vivo human measurements atop the intact epithelium. In the current experiments, a lower variance would have likely resulted in a statistically significant increase in measured stiffness after debridement. Whether measurements over intact epithelium provide data that are comparable to direct stromal measurements in human tissue is an important issue that remains to be resolved.
Partial-thickness corneal incisions not only reduced the wave velocity across the incision site but also produced significant changes in radial stiffness peripheral to the central incisions (Table 2). These changes were not uniform and varied with the depth of the incision and according to measurement orientation relative to the incision plane. A tendency toward increased stiffness across the entire vertical meridian (defined as parallel to the incision) is consistent with the concept that few collagen fibers are disrupted along a vector parallel to the incision and may be a biomechanical correlate to the refractive “coupling” effects39-41 commonly cited in incisional keratotomy. Although the analysis was not adequately powered to rule out a small reduction in cross-incisional velocity, tripling of the incision depth did not significantly reduce wave velocity further, suggesting that stiffness sampling is weighted toward the anterior 250 μm. Vexler et al24 reported a 1-mm sampling depth in skin with a 5.4-kHz wave frequency, but signal attenuation is a function of depth and will produce a gradient of sensitivity that is greatest near the anterior surface. Our finding that pre-incision measurements were not significantly different in the two meridia agrees with porcine data from Kampmeier et al42 demonstrating little anisotropy. Without information on globe orientation, however, we can conclude only that there are no differences between aggregate stiffness measurements in the central, radial, and circumferential porcine cornea. The ability to measure induced meridional anisotropy relative to the incision direction was clearly demonstrated, however, and illustrates the use of a directionally sensitive measurement technique. Lamellar relaxation effects and anisotropy are critical mechanical variables in astigmatic keratotomy as well as in PRK, PTK, and LASIK,9,10,21,43 and stiffness mapping according to the template proposed in Figure 2 may be useful for methodical investigation of these complex biomechanical relationships and their impact on corneal optical performance. Early results in separate donor globe experiments suggest that regional and directional differences in human tissue, unlike in porcine tissue, are significant according to the same measurement scheme.44
An unexpected and interesting finding relates to the significant increase in radial stiffness throughout the entire paracentral region (positions 3, 4, 5, and 6) when central incisions were deepened to 750 μm. Perhaps most unexpected was the observation that this stiffness increase occurred primarily in the meridian perpendicular to the incision, ie, along the axis of the transected collagen fibers. Central keratotomy or keratectomy has been proposed to relax the severed lamellae and reduce resistance to the stromal swelling pressure, which leads to increased peripheral stromal thickness via fluid expansion of the interlamellar space.9,10 Exaggeration of this response, along with continued volumetric expansion of the stromal matrix and centripetal retraction of collagen, may invoke stiffening of peripheral collagen that, while severed centrally, may participate in loading through interlamellar cohesion and branching fibrils that extend across multiple lamellar depths. Stated differently, this observation may relate to a viscoelastic stress-stiffening effect in the radial corneal periphery that is not seen after more superficial interventions. Circumferential limbal measurements, which capture stiffness components along a predominantly circumferential collagen fibril orientation, do not appear to be affected significantly by central keratotomy and may provide a potentially stable internal reference measurement for normalization of other regional measurements in longitudinal studies.
Cross-linking of human corneas with a dialdehyde agent led to dramatic increases in wave velocity and transcorneal IOP measurements despite a constant intravitreal pressure. A pressure of 30 mmHg was chosen because in these particular globes it provided excellent pressure stability over time without exceeding levels commonly encountered in ocular hypertension and glaucoma. Glutaraldehyde is a powerful and well-established agent for increasing the elastic modulus of collagenous materials, including cornea.29,30 Transcorneal IOP measurements such as pneumatonometry, TonoPen, and Goldmann applanation are dependent on the intrinsic corneal resistance to indentation in addition to the true IOP.3 Our current report may be the first to explicitly demonstrate that stiffening of the human cornea can produce an artifactual increase in measured IOP without significant alterations in true IOP or corneal thickness.
Edematous corneas such as those used in our cross-linking experiments have been shown to report lower pressures by applanation tonometry than normally hydrated corneas.45,46 This phenomenon may be due to a decreased ratio of stiff (collagenous) to viscous (fluid plus matrix) corneal components, which would tend to decrease effective corneal stiffness when tested by an applanation force or a propagating ultrasonic wave. Such a decrease in measured stiffness may occur without an actual change in the stiffness of the collagen component. The importance of the ratio of stiff to viscous corneal mass is supported by recent air-puff hysteresis measurements in patients with Fuchs' dystrophy,47 in whom reductions in hysteresis similar to those seen in keratoconus patients have been observed relative to a normal population. These changes most likely represent a relative “softening” due to increased fluid per unit volume and not due to an abnormality of collagen/matrix stiffness in endothelial dystrophy. Conversely, dehydration of edematous corneas during cross-linking could also increase apparent stiffness across the sampled cornea. In our experiment, modest corneal thickness decreases of 5% and 9% (in eye 1 and 2, respectively) are unlikely to account for wave velocity increases of 81% and 86%, and stiffening must therefore be primarily related to the cross-linking effects of glutaraldehyde. The marked IOP artifacts we demonstrated are less likely to be seen after clinical riboflavin/ultraviolet-A mediated corneal cross-linking because glutaraldehyde, particularly at the concentration used here, is a much more potent cross-linking agent.29
Recent epidemiologic analyses outside the setting of corneal edema have demonstrated that central corneal thickness is a powerful predictor of progression from ocular hypertension to glaucoma.48 A theoretical analysis by Liu and Roberts4 suggests that the elastic modulus is probably more influential than corneal thickness as a source of error in IOP measurement. The results of the Ocular Hypertension Treatment Study should be carefully considered in this context, where corneal thickness could owe its predictive value primarily to a certain level of covariance with corneal stiffness. Although corneal thickness and corneal stiffness are likely covariants in a general sense, the stiffness as measured by a propagating surface wave may be less affected by thickness in a porcine model because, as we demonstrate in the serial keratotomy experiments, the surface wave biases the sampling of properties toward the anterior cornea and appears to attenuate somewhere between 250 and 750 μm of depth. The sensitivity of surface wave elastometry to corneal thickness could potentially be greater in human corneas where the likelihood of sampling the less rigid posterior cornea and aqueous humor prior to significant attenuation is higher.
Although thicker corneas may be stiffer and thinner corneas less rigid in general, the two properties can vary independently. Using a tool capable of objective measurements of corneal stiffness, we demonstrated this in an artificial but dramatic way in the cross-linking experiment. The lack of a simple linear correction factor for IOP based on central corneal thickness alone49 may reflect a naturally occurring nonlinearity between thickness and material stiffness. Regression models for predicting true IOP based on measured IOP and central corneal thickness may benefit from addition of a third predictive variable such as central surface wave velocity that could account for currently unexplained variance in this critical variable. Further work with laboratory and clinical elastometry thus has important implications for assessing IOP and glaucoma risk after corneal refractive surgery,49,50 in keratoconus and other ectasias, and after UV-riboflavin collagen cross-linking for treatment of ectatic disease.51
One commercial device for quantifying corneal biomechanical properties has recently become available. The Ocular Response Analyzer (ORA; Reichert Inc, Depew, NY) was alluded to above and uses a high-speed air-puff applanation technique to quantify the dynamics of corneal deformation and recovery as an indicator of global corneal hysteresis.47 Studies of this viscoelastic parameter in normal, keratoconus, and post-LASIK patients are ongoing. This technique and the surface wave velocity method involve entirely different mechanical stimuli and therefore measure different and potentially complimentary mechanical properties. The wave propagation technique measures velocity along the long axes of collagen lamellae parallel to the line of measurement and samples a thickness of cornea related to the wave amplitude, whereas the air-puff technique measures the discrepancy in high-speed bending properties with loading and unloading axially perpendicular to the long axes of collagen lamellae. Both techniques acquire raw signals that are far more complex than the velocity or hysteresis indices presented to the end-user. Future work will seek to identify the most informative representations of these signals and to investigate their relationship to various properties of interest.
Practical attempts at measuring elastic properties provide approximations of the true elastic modulus.52 Although a precise definition of the relationship between surface wave velocity and the elastic modulus of the cornea is not attempted here, a first-approximation of the elastic modulus can be derived from the linear, isotropic, and homogeneous case where the elastic modulus (E) is the product of the density of the cornea (ρ) and the square of the wave velocity (V2).23 Given that the density of the porcine cornea has been measured as 1062±5 kg/m3,42 the corresponding approximation of the elastic modulus for a wave velocity of 120 m/s from our porcine experiments would be 15.3×106 kgm−1s−2 or 15.3 MPa. A similar approximation for the human corneas in this report would be 6 MPa based on an average wave speed of 80 m/s (Table 3) and a similar density assumption.
Using a uniaxial tensile testing approach, Nyquist found an elastic modulus of 16 MPa in excised porcine corneal strips,53 which is similar to the 15.3 MPa we estimate for the simple linear isotropic case. Because Nyquist used stresses higher than those encountered under physiological loading (15 mmHg of distributed IOP in our experiments), we expect that the simple modulus calculation above results in an overestimation of the true modulus. Comparable values for human corneal elastic modulus measured by uniaxial extensiometry were obtained with an IOP stress equivalent of 10 mmHg by Hoeltzel et al54 (0.34 MPa) and Woo et al55 (0.54 MPa) and are approximately one order of magnitude lower, again suggesting that the linear, isotropic, homogeneous assumption leads to an overestimation of elastic modulus in this context. Efforts to better characterize the relationship of surface wave velocity to the elastic modulus are underway.
Although representative estimates of the elastic modulus are desirable for computational models using formal constitutive material relationships, a great need also exists for useful approximations of corneal stiffness that can be used simply to detect differences or changes in stiffness throughout the spectrum of applications discussed earlier in this report. Sonic wave propagation can provide a meaningful and repeatable measure of directional and regional stiffness changes resulting from relevant weakening and strengthening interventions. The precision of measurements in corneal tissue and in the synthetic elastomer—which we recommend including in every session as a potential normalizing factor—allows reasonable determinations of relative stiffness between regions of a given cornea, between different corneas, and in the same cornea before and after an intervention. Reproducibility and sensitivity are important if the tool is to facilitate systematic study of wave velocity as a predictor of surgical response, ectasia risk, and true IOP. In practice, an internally consistent measurement obtained from the tissue of interest is a more valuable predictor of mechanical behavior than a modulus extrapolated from experiments in noncomparable tissue. In vivo application requires additional attention to the role of the tear film, potential confounding influences from the epithelium and topical anesthetic, and optimization of probe frequency for appropriate corneal depth sampling.
Acknowledgments
Supported in part by a Research to Prevent Blindness Challenge Grant to the Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine, NIH 8K12 RR023264 Multidisciplinary Clinical Research Career Development Programs Grant (Dupps), and NIH 1L30 EY017803-01 (Dupps).
REFERENCES
- 1.Comaish IF, Lawless MA. Progressive post-LASIK keratectasia: biomechanical instability or chronic disease process? J Cataract Refract Surg. 2002;28:2206–2213. doi: 10.1016/s0886-3350(02)01698-x. [DOI] [PubMed] [Google Scholar]
- 2.Dupps WJ., Jr. Biomechanical modeling of corneal ectasia. J Refract Surg. 2005;21:186–190. doi: 10.3928/1081-597X-20050301-15. [DOI] [PubMed] [Google Scholar]
- 3.Friedenwald JS. Contribution to the theory and practice of tonometry. Am J Ophthalmol. 1937;20:985–1024. [Google Scholar]
- 4.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Gasset AR, Dohlman CH. The tensile strength of corneal wounds. Arch Ophthalmol. 1968;79:595–602. doi: 10.1001/archopht.1968.03850040597020. [DOI] [PubMed] [Google Scholar]
- 6.Hjortdal JO, Moller-Pedersen T, Ivarsen A, Ehlers N. Corneal power, thickness, and stiffness: results of a prospective randomized controlled trial of PRK and LASIK for myopia. J Cataract Refract Surg. 2005;31:21–29. doi: 10.1016/j.jcrs.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 7.Bryant MR, Szerenyi K, Schmotzer H, McDonnell PJ. Corneal tensile strength in fully healed radial keratotomy wounds. Invest Ophthalmol Vis Sci. 1994;35:3022–3031. [PubMed] [Google Scholar]
- 8.Orwin EJ, Borene ML, Hubel A. Biomechanical and optical characteristics of a corneal stromal equivalent. J Biomech Eng. 2003;125:439–444. doi: 10.1115/1.1589773. [DOI] [PubMed] [Google Scholar]
- 9.Dupps WJ, Jr, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg. 2001;17:658–669. doi: 10.3928/1081-597X-20011101-05. [DOI] [PubMed] [Google Scholar]
- 10.Roberts C. The cornea is not a piece of plastic. J Refract Surg. 2000;16:407–413. doi: 10.3928/1081-597X-20000701-03. [DOI] [PubMed] [Google Scholar]
- 11.Katsube N, Wang R, Okuma E, Roberts C. Biomechanical response of the cornea to phototherapeutic keratectomy when treated as a fluid-filled porous material. J Refract Surg. 2002;18:S593–S597. doi: 10.3928/1081-597X-20020901-19. [DOI] [PubMed] [Google Scholar]
- 12.Guirao A. Theoretical elastic response of the cornea to refractive surgery: risk factors for keratectasia. J Refract Surg. 2005;21:176–185. doi: 10.3928/1081-597X-20050301-14. [DOI] [PubMed] [Google Scholar]
- 13.Hanna KD, Jouve FE, Waring GO., III Preliminary computer simulation of the effects of radial keratotomy. Arch Ophthalmol. 1989;107:911–918. doi: 10.1001/archopht.1989.01070010933044. [DOI] [PubMed] [Google Scholar]
- 14.Hanna KD, Jouve FE, Waring GO, III, Ciarlet PG. Computer simulation of arcuate and radial incisions involving the corneoscleral limbus. Eye. 1989;3:227–239. doi: 10.1038/eye.1989.32. [DOI] [PubMed] [Google Scholar]
- 15.Vito RP, Shin TJ, McCarey BE. A mechanical model of the cornea: the effects of physiological and surgical factors on radial keratotomy surgery. Refract Corneal Surg. 1989;5:82–88. [PubMed] [Google Scholar]
- 16.Pinsky PM, Datye DV. A microstructurally-based finite element model of the incised human cornea. J Biomech. 1991;24:907–922. doi: 10.1016/0021-9290(91)90169-n. [DOI] [PubMed] [Google Scholar]
- 17.Pinsky PM, Datye DV. Numerical modeling of radial, astigmatic, and hexagonal keratotomy. Refract Corneal Surg. 1992;8:164–172. [PubMed] [Google Scholar]
- 18.Bryant MV. Design of Keratorefractive Surgical Procedures: Radial Keratotomy. American Society of Mechanical Engineering; New York, NY: 1989. [Google Scholar]
- 19.Vito RP, Carnell PH. Finite element based mechanical models of the cornea for pressure and indenter loading. Refract Corneal Surg. 1992;8:146–151. [PubMed] [Google Scholar]
- 20.Anderson K, El-Sheikh A, Newson T. Application of structural analysis to the behaviour of the cornea. J R Soc Interface. 2004;1:3–15. doi: 10.1098/rsif.2004.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant MR, McDonnell PJ. Constitutive laws for biomechanical modeling of refractive surgery. J Biomech Eng. 1996;118:473–481. doi: 10.1115/1.2796033. [DOI] [PubMed] [Google Scholar]
- 22.Potts RO, Chrisman DA, Jr, Buras EM., Jr The dynamic mechanical properties of human skin in vivo. J Biomech. 1983;16:365–372. doi: 10.1016/0021-9290(83)90070-2. [DOI] [PubMed] [Google Scholar]
- 23.Lempriere BM. Ultrasound and Elastic Waves. Academic Press; San Diego, Calif: 2002. [Google Scholar]
- 24.Vexler A, Polyansky I, Gorodetsky R. Evaluation of skin viscoelasticity and anisotropy by measurement of speed of shear wave propagation with viscoelasticity skin analyzer. J Invest Dermatol. 1999;113:732–739. doi: 10.1046/j.1523-1747.1999.00751.x. [DOI] [PubMed] [Google Scholar]
- 25.Smolek MK. Interlamellar cohesive strength in the vertical meridian of human eye bank corneas. Invest Ophthalmol Vis Sci. 1993;34:2962–2969. [PubMed] [Google Scholar]
- 26.Smolek MK, McCarey BE. Interlamellar adhesive strength in human eyebank corneas. Invest Ophthalmol Vis Sci. 1990;31:1087–1095. [PubMed] [Google Scholar]
- 27.Meek KM, Newton RH. Organization of collagen fibrils in the corneal stroma in relation to mechanical properties and surgical practice. J Refract Surg. 1999;15:695–699. doi: 10.3928/1081-597X-19991101-18. [DOI] [PubMed] [Google Scholar]
- 28.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–2258. [PubMed] [Google Scholar]
- 29.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 30.Dupps WJ., Jr . Doctoral thesis. The Ohio State University; Columbus, Ohio: 1998. Chemo-mechanical modification of the corneal response to photokeratectomy. [Google Scholar]
- 31.Wang H, Prendiville PL, McDonnell PJ, Chang WV. An ultrasonic technique for the measurement of the elastic moduli of human cornea. J Biomech. 1996;29:1633–1636. [PubMed] [Google Scholar]
- 32.Liu J, Roberts CJ. An ultrasound propagation model for characterizing biomechanical properties of ocular tissue; Presented at: Second International Conference on the Ultrasonic Measurement and Imaging of Tissue Elasticity; Corpus Christi, Tex. Oct 12−15, 2003. [Google Scholar]
- 33.Potts RO, Buras EM, Jr, Chrisman DA., Jr Changes with age in the moisture content of human skin. J Invest Dermatol. 1984;82:97–100. doi: 10.1111/1523-1747.ep12259203. [DOI] [PubMed] [Google Scholar]
- 34.Mridha M, Odman S, Oberg PA. Mechanical pulse wave propagation in gel, normal and oedematous tissues. J Biomech. 1992;25:1213–1218. doi: 10.1016/0021-9290(92)90077-e. [DOI] [PubMed] [Google Scholar]
- 35.Pereira JM, Mansour JM, Davis BR. Analysis of shear wave propagation in skin; application to an experimental procedure. J Biomech. 1990;23:745–751. doi: 10.1016/0021-9290(90)90021-t. [DOI] [PubMed] [Google Scholar]
- 36.Nizet JL, Pierard-Franchimont C, Pierard GE. Influence of body posture and gravitational forces on shear wave propagation in the skin. Dermatology. 2001;202:177–180. doi: 10.1159/000051629. [DOI] [PubMed] [Google Scholar]
- 37.Davis BR, Bahniuk E, Young JK, Barnard CM, Mansour JM. Age-dependent changes in the shear wave propagation through human skin. Exp Gerontol. 1989;24:201–210. doi: 10.1016/0531-5565(89)90011-9. [DOI] [PubMed] [Google Scholar]
- 38.Gorodetsky R, Lotan C, Piggot K, Pierce LJ, Polyansky I, Dische S, Saunders MI, Lichter AS, Vexler A. Late effects of dose fractionation on the mechanical properties of breast skin following post-lumpectomy radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:893–900. doi: 10.1016/s0360-3016(99)00257-6. [DOI] [PubMed] [Google Scholar]
- 39.Rowsey JJ, Fouraker BD. Corneal coupling principles. Int Ophthalmol Clin. 1996;36:29–38. doi: 10.1097/00004397-199603640-00006. [DOI] [PubMed] [Google Scholar]
- 40.Thornton SP. Astigmatic keratotomy: a review of basic concepts with case reports. J Cataract Refract Surg. 1990;16:430–435. doi: 10.1016/s0886-3350(13)80795-x. [DOI] [PubMed] [Google Scholar]
- 41.Duffey RJ, Jain VN, Tchah H, Hofmann RE, Lindstrom RL. Paired arcuate keratotomy. A surgical approach to mixed and myopic astigmatism. Arch Ophthalmol. 1988;106:1130–1135. doi: 10.1001/archopht.1988.01060140286043. [DOI] [PubMed] [Google Scholar]
- 42.Kampmeier J, Radt B, Birngruber R, Brinkmann R. Thermal and biomechanical parameters of porcine cornea. Cornea. 2000;19:355–363. doi: 10.1097/00003226-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Roberts C. Biomechanics of the cornea and wavefront-guided laser refractive surgery. J Refract Surg. 2002;18:S589–S592. doi: 10.3928/1081-597X-20020901-18. [DOI] [PubMed] [Google Scholar]
- 44.Dupps WJ, Krueger RR, Jeng BH. Regional stiffness of human donor corneas measured by sonic wave elastometry. Invest Ophthalmol Vis Sci. 2006;47:E-abstract 1335. [Google Scholar]
- 45.Simon G, Small RH, Ren Q, Parel JM. Effect of corneal hydration on Goldmann applanation tonometry and corneal topography. Refract Corneal Surg. 1993;9:110–117. [PubMed] [Google Scholar]
- 46.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 47.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 48.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 49.Brandt JD. Corneal thickness in glaucoma screening, diagnosis, and management. Curr Opin Ophthalmol. 2004;15:85–89. doi: 10.1097/00055735-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Munger R, Dohadwala AA, Hodge WG, Jackson WB, Mintsioulis G, Damji KF. Changes in measured intraocular pressure after hyperopic photorefractive keratectomy. J Cataract Refract Surg. 2001;27:1254–1262. doi: 10.1016/s0886-3350(01)00971-3. [DOI] [PubMed] [Google Scholar]
- 51.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 52.Samani A, Bishop J, Luginbuhl C, Plewes DB. Measuring the elastic modulus of ex vivo small tissue samples. Phys Med Biol. 2003;48:2183–2198. doi: 10.1088/0031-9155/48/14/310. [DOI] [PubMed] [Google Scholar]
- 53.Nyquist GW. Rheology of the cornea: experimental techniques and results. Exp Eye Res. 1968;7:183–188. doi: 10.1016/s0014-4835(68)80064-8. [DOI] [PubMed] [Google Scholar]
- 54.Hoeltzel DA, Altman P, Buzard K, Choe K. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J Biomech Eng. 1992;114:202–215. doi: 10.1115/1.2891373. [DOI] [PubMed] [Google Scholar]
- 55.Woo SL, Kobayashi AS, Schlegel WA, Lawrence C. Nonlinear material properties of intact cornea and sclera. Exp Eye Res. 1972;14:29–39. doi: 10.1016/0014-4835(72)90139-x. [DOI] [PubMed] [Google Scholar]