Abstract
Muscle injury or modified muscle use can stimulate muscle invasion by leucocytes that have the potential to increase tissue damage or promote tissue growth and repair. In the present investigation, we examined the role of macrophages in muscle injury, repair and regeneration during modified muscle loading. Weight-bearing was removed from the hindlimbs of mice for 10 days followed by reloading through normal ambulation. During the unloading period, soleus muscle fibre cross-section decreased by 38%. Prior to the onset of reloading, mice received a series of intraperitoneal injections of anti-F4/80, which binds a mouse macrophage surface antigen. Although anti-F4/80 injections did not affect macrophage numbers in soleus muscles at 2 days of reloading, macrophages were reduced by 86% at 4 days of reloading. Muscle membrane lysis during the reloading period did not differ at 2 days of reloading between anti-F4/80-treated mice and mice that received isotype control antibody. However, control animals showed large decreases in the number of fibres with membrane lesions at 4 days of reloading, but this membrane repair did not occur in macrophage-depleted mice. Macrophage-depletion also reduced muscle regeneration (indicated by central nucleation) and satellite cell differentiation (indicated by reductions in MyoD-expressing satellite cells) and prevented growth of muscle fibres that normally occurred in control animals between days 2 and 4 of reloading. These findings collectively show that macrophages play a significant role in muscle fibre membrane repair, regeneration and growth during increased muscle use after a period of atrophy.
Interactions between myeloid cells and skeletal muscle cells can influence muscle cell proliferation, differentiation and injury through mechanisms that are only beginning to be understood. In vitro and in vivo findings offer strong evidence that macrophages can increase muscle membrane lysis, and presumably thereby increase muscle injury (Wehling et al. 2001; Nguyen & Tidball, 2003a, 2003b). Membrane lysis by macrophages in vitro occurs through a nitric oxide (NO)-dependent and superoxide-independent process (Nguyen & Tidball, 2003a). In addition, NO-mediated lysis of muscle membranes by macrophages in vitro is exacerbated by the presence of neutrophils (Nguyen & Tidball, 2003a), which suggests that signalling between myeloid cell populations can affect their cytotoxicity. These cytotoxic interactions between myeloid cells and muscle are also apparently modulated by muscle-derived factors, at least in vitro, where muscle-derived factors increase NO release by macrophages (Nguyen & Tidball, 2003a).
Macrophages are also able to promote muscle growth and repair. In vitro findings show that conditioned media from peritoneal macrophages or macrophage cell lines can increase proliferation of myoblasts in culture and elevate the proportion of myoblasts that express MyoD (Cantini & Carraro, 1995; Cantini et al. 2002), which indicates a role for macrophage-derived factors in muscle growth and differentiation. In vivo observations may also support a positive role for macrophages in muscle growth and repair. Muscle repair by transplanted whole-muscle grafts is diminished if the graft recipients are irradiated before transplantation (Lescaudron et al. 1999), which reflects a role for proliferative cells, such a macrophages, in muscle regeneration. More recent findings have shown that null mutation of cyclooxygenase-2 (COX-2) or administration of COX-2 inhibitors can slow muscle regeneration and reduce myoblast proliferation after acute injury of muscle (Bondesen et al. 2004). COX-2 null mutants also showed less macrophage invasion of injured muscle during regeneration (Bondesen et al. 2004), which may indicate that macrophages normally promote muscle cell proliferation and muscle regeneration following injury. Alternatively, COX-2 may have a direct effect on muscle cells to affect proliferation and repair.
The apparently conflicting roles of macrophages in promoting muscle injury and repair may reflect the functions of distinct macrophage subpopulations in muscle. Several investigations have supported the potentially dichotomous role of macrophage subpopulations by examining the time courses of macrophage invasion, muscle fibre damage and muscle repair following modified muscle use. During periods of increased muscle use that are sufficient to cause muscle membrane lysis and muscle inflammation, muscle is initially invaded by a phagocytic population of macrophages that can enter and degrade the contents of injured muscle fibres (Krippendorf & Riley, 1993; St Pierre & Tidball, 1994; Tidball et al. 1999). These macrophages reach peak concentrations in the muscle at 2 days following increased muscle loading, and then rapidly decline in numbers (St Pierre & Tidball, 1994). Most lesions of the muscle membrane that are caused by muscle reloading occur during this first 2 day period (Tidball et al. 1999). A second, non-phagocytic population (McLennan, 1993) invades the muscle and reaches peak concentration at 4 days following increased loading, but remains elevated for at least several days after muscle loading is increased (Krippendorf & Riley, 1993. St Pierre & Tidball, 1994; Tidball et al. 1999). This second, non-phagocytic population is mostly distributed near regenerative fibres (St Pierre & Tidball, 1994), suggesting their potential role in muscle regeneration. Furthermore, peritoneal macrophages that are phenotypically similar to the non-phagocytic population in injured muscle have been shown to release in vitro unknown factors that can promote myoblast proliferation (Massimino et al. 1997).
In the present investigation, we have tested whether the late invading population of macrophages affects muscle membrane lysis, membrane repair, satellite cell activation, muscle regeneration or muscle fibre growth during a period of increased muscle loading that is imposed after a 10 day period of muscle unloading. We have developed a protocol for antibody depletion of macrophages that reduces the concentration of macrophages after 2–4 days of increased loading, but does not affect macrophage numbers during earlier stages of increased muscle loading. Our findings show that the selective depletion of macrophages at the 2–4 day time point prevents repair of the muscle cell membrane that normally occurs at this time and prevents muscle fibre growth and regeneration, which usually occur between 2 and 4 days of increased muscle use. These findings show that the late-invading macrophage population can contribute significantly to muscle repair, growth and regeneration in vivo.
Methods
Animals
All experiments involving animals were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of California, Los Angeles Institutional Animal Care and Use Committee. C57BL/6J mice were obtained from the Jackson Laboratories (Bar Harbour, ME, USA) and were 5 months of age at the time of experimentation. Mice were maintained in an accredited animal care facility and examined daily for signs of distress, injury or disease. At the end of experimentation, mice were killed by intraperitoneal injection with an overdose of sodium pentobarbital, according to the Panel on Euthanasia of the American Veterinary Medical Association.
Hindlimb muscle unloading and reloading
Muscle injury and inflammation were induced by subjecting mice to 10 days of muscle unloading of both hindlimbs, followed by reloading for 2 days or 4 days by normal weight bearing. An apparatus that was a modification of that described by Morey-Holton & Globus (2002) was used for hindlimb unloading. Muscle unloading by this technique produces approximately a 40% mass loss of the soleus muscle in a 10 day period (Thomason & Booth, 1990) and approximately a 30% reduction in fibre cross-sectional area. Muscle reloading when returned to normal ambulation causes muscle inflammation, fibre injury and membrane lesions in soleus muscle fibres (Krippendorf & Riley, 1993; St Pierre & Tidball, 1994; Kasper, 1995; Tidball et al. 1999). Mice received either anti-F4/80 injections or injections of isotype control antibody followed by either 0, 2 or 4 days of reloading after hindlimb unloading. There were five mice in each anti-F4/80 or isotype control IgG group at each time point. The ‘unloaded only’ groups consisted of mice injected with anti-F4/80 or isotype control antibody that were subjected to hindimb unloading for 10 days and then immediately killed for tissue collection, without experiencing reloading. The ‘ambulatory control’ group consisted of five control mice that experienced normal cage activity until killed for tissue collection. After the animal was killed, soleus muscles were rapidly dissected. One soleus muscle from each animal was rapidly frozen in isopentane, and used for immunohistochemical analysis and Western blot analysis. The second soleus from each animal was used for assessment of muscle membrane damage.
Macrophage depletion protocol
Anti-F4/80 was prepared by ammonium sulphate precipitation of immunoglobulins from F4/80 hybridoma cultures (ATCC). The precipitated immunoglobulins were resuspended in 50 mm sodium phosphate buffer pH 7.2 containing 150 mm sodium chloride (PBS), sterile filtered and then the IgG concentration was assayed by enzyme-linked immunosorbent assay. Mice received intraperitoneal injections of 100 μg anti-F4/80 IgG or isotype control IgG (rat IgG2b; BD Pharmingen, San Diego, CA, USA) every 24 h, beginning on day 7 of hindlimb unloading until the day the animal was killed.
Immunohistochemistry
Frozen, cross-sections were taken from the midbelly of one soleus muscle from each animal and used for immunohistochemical analysis. The 10 μm thick sections were fixed in acetone and then immunolabelled for macrophages using using rat anti-mouse F4/80, or for satellite cells using mouse anti-MyoD (clone 5.8A; RDI, Flanders NJ, USA), or mouse anti-Pax-7 (clone P3U1; Developmental Studies Hybridoma Bank, Iowa City IA, USA). Sections probed with anti-F4/80 were processed as previously described (Wehling et al. 2001) and immunoreactive cells were identified using a biotinylated mouse anti-rat IgG second antibody and horseradish peroxidase conjugated avidin before reaction with aminoethylcarbonyl (Vector). Sections treated with antibodies generated by mouse hybridomas were processed similarly, although endogenous IgGs were blocked using a MOM kit (Vector) and the second antibody was a rat anti-mouse IgG. The total volume of each section was determined by measuring the area of each section using a stereological, point-counting technique (Spencer et al. 2001), and then multiplying that value by the section thickness. The concentrations of immunolabelled cells were expressed as the number of cells per volume of each section. One-way analysis of variance was used to test whether variation between groups was significant at P < 0.05. Bartlett's test for homogeneity of variances was used to test whether all experimental groups came from populations with equal standard deviations. The Bonferonni multiple comparisons test was used to test for differences between pairs of experimental groups with P < 0.05.
Assays of muscle membrane injury
Injuries to soleus muscle fibre membranes were assayed by measuring the relative concentration of the fluorescent, extracellular tracer dye, procion orange, in the cytoplasm of muscle fibres. Procion orange dye solutions remain in the extracellular space unless membrane lesions are present. One soleus from each experimental or control mouse was incubated in 0.5% procion orange dye solution in Krebs-Ringer solution for 1 h followed by washes with Krebs-Ringer solution. The soleus muscles were then frozen in isopentane and serial cross-sections for each muscle were cut at 10 μm thickness. Fibre membrane injury was assessed using two assays. In the first, the number of brightly fluorescent, injured fibres in each section taken from the midbelly of each muscle was expressed as a percentage of the total fibres that were present in the section. The size of the lesions in these brightly fluorescent fibres was also determined by following each fibre through serial sections of the muscle. In the second assay, the fluorescence intensity of each, individual fibre in each muscle cross-section was measured in an 8 μm diameter, circular area that was sampled at the centre of each fibre using a digital imaging system (Bioquant, Nashville, TN, USA). Fluorescence intensity values for each fibre were attained below signal saturation levels and were corrected for background levels, by measuring the signal from an area of the slide that contained no tissue, and subtracting that background value from the cytosolic fluorescence measurements.
Assay for muscle membrane remodelling
Muscle membrane remodelling was assessed by comparing the relative concentrations of dysferlin in extracts of experimental and control soleus muscles by Western blot analysis. Previous investigations have established that dysferlin participates in the repair of muscle fibre membranes following acute, muscle injury (Lennon et al. 2003), as well as muscle membrane remodelling during muscle atrophy (Chopard et al. 2005). Samples were homogenized in a Dounce homogenizer in reducing sample buffer (80 mm Tris, pH 6.8, containing 0.1 m dithiothreitol and 70 mm SDS), and then boiled for 1 min and centrifuged to remove particulate material. The protein concentration of the supernatant fraction was measured (Minamide & Bamburg, 1990) and 30 mg each sample was loaded on 10% polyacrylamide gels (Laemmli, 1970), and then transferred electrophoretically to nitrocellulose (Burnette, 1981). Protein blots were incubated with a mouse anti-dysferlin (clone Ham3/17B2; Novocastra, Newcastle-upon-Tyne, UK) diluted 1 : 20 in 50 mm Tris, pH 7.6, containing 150 mm NaCl, 0.1% NaN3, 0.05% Tween 20, and 3% bovine serum albumin. After washing with buffer, the blots were incubated with a second antibody conjugated to horseradish peroxidase and the bound antibody was detected by enhanced chemiluminescence (Amersham). After digitally recording the chemiluminescent signal, the blots were stripped of antibody by incubating in them 62.5 mm Tris at pH 6.7 containing 2% SDS and 100 mm β-mercaptoethanol and then reprobed with rabbit anti-chicken desmin (Sigma) followed by similar processing and incubation with a second antibody to rabbit IgG that was conjugated to horseradish peroxidase and then assayed by enhanced chemiluminescence. The desmin labelling was used to assess uniformity of loading, and to normalize the dysferlin data relative to muscle protein.
Assay for muscle fibre regeneration
Myonuclei that are distributed along the longitudinal, central axes of muscle fibres, called central nuclei, are morphological markers of fibres undergoing regeneration (Bigard et al. 1975). The number of muscle fibres in complete cross-sections of soleus muscles from experimental and control animals was counted and expressed as a percentage of the total number of fibres in the muscle cross-section to provide an index of muscle regeneration. Each value is expressed as the mean ± s.e.m.
Measurement of muscle fibre cross-sectional area
Muscle fibre cross-sectional area was measured for every fibre in complete cross-sections of each soleus muscle using a digital imaging system (Bioquant). Muscle atrophy or growth was assessed by measuring changes in fibre cross-sectional area (Nguyen & Tidball, 2003b).
Results
Anti-F4/80 treatments significantly reduce macrophage concentrations in reloaded muscle by 4 days of reloading
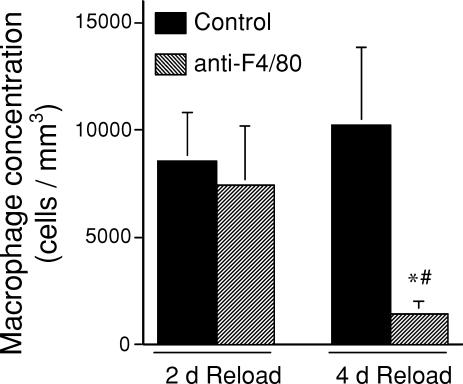
Immunohistochemical analysis of the concentration of F4/80-expressing macrophages in soleus muscles at the end of a 10 day period of muscle unloading, and then at 2 or 4 days of reloading confirms that muscle reloading causes macrophage invasion of soleus muscles. As previously reported (Krippendorf & Riley, 1993; St Pierre & Tidball, 1994), macrophages that invaded at early stages of reloading tended to be most concentrated near damaged fibres, and occasionally were observed to have infiltrated fibres. Macrophages that invaded at later stages, such as after 4 days of reloading, were distributed more homogeneously within the perimysium, and did not invade muscle fibres. Macrophage concentrations in soleus muscle of control mice are relatively low after 10 days of unloading (817 ± 142 cells mm−3, n = 5), but after 2 days of reloading they increase significantly (8590 ± 2212 cells mm−3, n = 5), and remain elevated after 4 days of reloading (10224 ± 3619 cells mm−3) (Fig. 1). Our anti-F4/80 treatment protocol did not cause a significant reduction in macrophage concentrations in soleus muscles after 10 days of unloading (721 ± 212 cells mm−3, n = 5) or at 2 days of reloading (7470 ± 2675 cells mm−3, n = 5), compared to isotype control IgG-treated mice. However, anti-F4/80 treatments caused an 86% reduction in macrophages in soleus muscles at the 4 day reloading time point (1462 ± 503 cells mm−3, n = 5) (Fig. 1).
Figure 1. Macrophage concentrations in soleus muscles of mice subjected to 10 days of hindlimb muscle unloading, followed by muscle reloading during normal ambulation.
Bars show s.e.m. *Significantly different from the isotype IgG control group during the same period of reloading; #significantly different from the 2 day reloaded group that received the same experimental treatment at P < 0.05.
Macrophage depletion between day 2 and day 4 of muscle reloading prevents muscle membrane repair during reloading
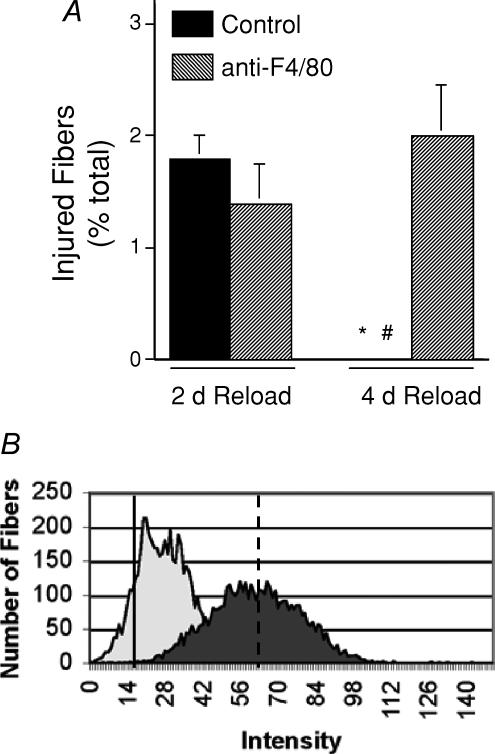
We identified muscle fibres with membrane lesions by the presence of the extracellular marker dye procion orange in the cytoplasm of the reloaded muscle fibres. The proportion of injured fibres that contained high concentrations of procion orange in the fibres, as indicated by bright cytosolic fluorescence observed by epifluorescence microscopy, did not differ significantly between anti-F4/80-treated mice and isotype control IgG-treated mice after 2 days of muscle reloading (non-depleted, 1.8 ± 0.2% injured, n = 5; F4/80 depleted, 1.4 ± 0.35% injured, n = 5) (Fig. 2). Inspection of the distribution of injured fibres in serial cross-sections of muscles that were reloaded for 2 days revealed no preferential distribution of lesions relative to the site at which the section was taken. Serial sections also showed that lesions were focal, and the high concentration of procion orange at lesion sites did not cause bright fluorescence along the entire length of injured fibres. The size of lesions was determined in a total of 46 fibres identified in six soleus muscles that experienced 2 days of reloading, which showed that foci of injury are 126 ± 76.0 μm in length. The typical, individual lesion formed 4.2 ± 2.5% of the total fibre length.
Figure 2. Macrophage depletion increases muscle fibre membrane lesions in the soleus muscles of mice subjected to 10 days of hindlimb unloading, followed by 4 days of muscle reloading.
A, data are the percentage of total fibres in the cross-sections of entire soleus muscles that were brightly fluorescent following procion orange treatment. Bars show s.e.m. *Significantly different from the anti-F4/80-injected group at the same stage of reloading; #significantly different from the 2-day reloaded group that received the same antibody treatment. B, the peaks show the aggregate data for measurements of intracellular fluorescence for all fibres in cross-sections of the entire soleus muscle from all mice in each treatment group. A rightward shift of peaks on the abscissa indicates an increase in fibres with membrane lesions. Note that the background fluorescence set at intensity = 0 was determined by measuring fluorescence at a region of the microscope slide where there was no tissue. Thus, even non-injured, ambulatory fibres displayed a small positive value for fluorescence. The dark peak shows data from anti-F4/80-treated mice after 4 days of reloading. The light peak shows data from isotype control antibody-treated mice after 4 days of reloading. Solid line shows mean fluorescence intensity of fibres from ambulatory animals. Dashed line shows mean fluorescence of fibres from isotype control IgG- and anti-F4/80-treated fibres after 2 days reloading, which did not differ between the two groups.
Although anti-F4/80 treatments had no significant effect on the proportion of fibres with membrane damage at 2 days of reloading, F4/80 depletions had a great effect at 4 days of reloading. No brightly flourescent fibres were observed in mice from which macrophages were not depleted, indicating that muscle membrane repair is a significant process between days 2 and 4 of reloading. However, macrophage-depleted mice at 4 days of reloading showed levels of fibre injury that did not differ significanctly from those at 2 days of reloading in either isotype-control or F4/80-treated mice (Fig. 2); this indicates that macrophages may promote membrane repair that occurs between days 2 and 4 of reloading.
Because measuring cell damage by determining the proportion of fibres that are brightly fluorescent after incubation in procion orange solution provides a large underestimate of the number of fibres with membrane damage (Nguyen & Tidball, 2003b), we also assayed the intracellular fluorescence of all fibres in soleus muscle cross-sections. These data were then expressed as a frequency distribution of fluorescence intensity for all soleus muscle fibres in anti-F4/80-treated and isotype control IgG-treated muscles (Fig. 2B). The mean fluorescence intensity did not differ between the anti-F4/80- or isotype IgG-treated groups after either 10 days of hindlimb unloading or after 2 days of reloading. However, after 4 days of reloading, the mean fluorescence intensity in the isotype control IgG group was significantly less than the anti-F4/80 group. These findings show that macrophage depletion prevented the repair of membrane damage that normally occurs between 2 and 4 days of muscle reloading.
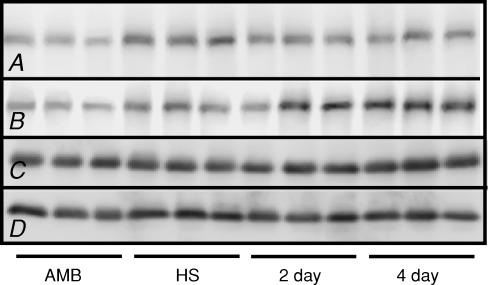
We further tested whether macrophages may play a role in membrane remodelling during reloading by assaying for dysferlin, a membrane-associated protein that has been implicated in muscle membrane turnover and repair (Bansal et al. 2003). Western blots show that soleus muscles from mice that were not treated with anti-F4/80 contained higher concentrations of dysferlin after 10 days of muscle unloading compared with levels in ambulatory control muscles (Fig. 3). No further increase in dysferlin in the isotype control-treated soleus muscles was evident during the reloading period. Anti-F4/80-treated mice showed a similar increase in dysferlin in soleus muscles during unloading. However, further increases in dysferlin concentration occurred in soleus muscles of macrophage-depleted mice at 4 days of reloading (Fig. 3), which suggests that in the absence of macrophages, muscle membrane turn-over and repair persist.
Figure 3. Western blot showing that dysferlin expression remained elevated after 4 days of reloading in the soleus muscles of mice that were macrophage depleted.
Each lane was loaded with 30 μg total protein. Row A, anti-dysferlin blot of muscle extracts from non-depleted mice. Row B, anti-dysferlin blot of muscle extracts from macrophage depleted mice. Row C, same blot as shown in row A, but reprobed with anti-desmin to show relative quantities of desmin in each lane, as a loading control. Row D, same blot as shown in row B, but reprobed with anti-desmin to show relative quantities of desmin in each lane, as a loading control. AMB, ambulatory controls; HS, hindlimb suspended only; 2 day, 2 day reloaded; 4 day, 4 day reloaded.
Macrophage depletion between day 2 and day 4 of muscle reloading reduces muscle regeneration and satellite cell differentiation
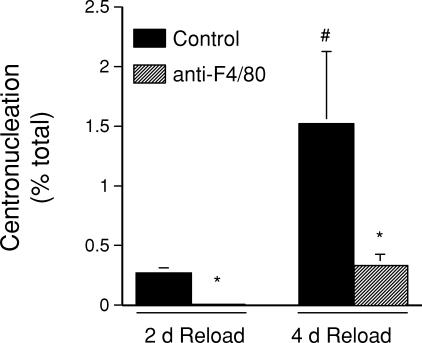
Centronucleation of muscle fibres was used as an index of muscle regeneration during muscle reloading. Isotype control IgG-treated animals showed more than a five-fold increase in the percentage of central-nucleated fibres in muscle cross-sections of 4 day reloaded muscle than in 2 day reloaded muscle (Fig. 4). However, anti-F4/80 treatments greatly reduced the occurrence of centronucleation, so that at 4 days of reloading the level of centronucleation in macrophage-depleted muscle was 77% less than in non-depleted muscles. This finding indicates that macrophages promote muscle regeneration during the period between 2 and 4 days of reloading.
Figure 4. The increase in regenerative fibres in soleus muscles that occurs between 2 and 4 days of reloading does not occur in macrophage-depleted mice.
The percentage of total muscle fibres in muscle cross-sections that were centrally nucleated was used as an index of regeneration. *Significantly different from control at P < 0.05; #significantly different from treatment-matched 2 day sample at P < 0.05.
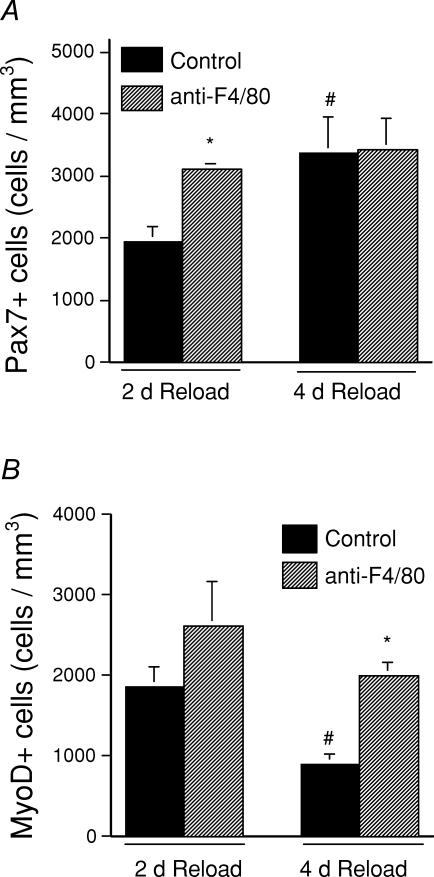
Quantitative immunohistochemistry of Pax7-expressing cells was used to determine total satellite cell concentrations because Pax7 is expressed by quiescent and proliferating satellite cells in vivo (Seale & Rudnicki, 2000). After 2 days of reloading, the concentration of Pax7-expressing cells was significantly higher in anti-F4/80-treated soleus muscles than in isotype control-treated soleus muscles (anti-F4/80 depleted, 3110 ± 88 Pax7 cells mm−3, n = 5; non-depleted, 1946 ± 247 Pax7 cells mm−3, n = 5). Although there is not a straightforward interpretation of this finding, it suggests that opsonization of the macrophages by the anti-F4/80 may have influenced macrophage function before there were quantitative differences in macrophage number in the antibody-treated animals. However, the concentrations of Pax7 cells in soleus muscles of the two treatment groups did not differ at 4 days of reloading (F4/80 depleted, 3428 ± 505 Pax7 cells mm−3, n = 5; non-depleted, 3377 ± 573 Pax7 cells mm−3, n = 5) (Fig. 5A).
Figure 5. Macrophage depletion affects the proliferation and differentiation of satellite cells during soleus muscle reloading following hindlimb suspension.
A, the number of satellite cells in macrophage-depleted soleus muscle is significantly greater than in non-depleted muscle at 2 days of reloading. B, MyoD-positive satellite cells decrease during the interval between 2 and 4 days of reloading in non-depleted but not in macrophage-depleted soleus muscles. *Significantly different from control at P < 0.05; #significantly different from treatment-matched 2 day sample at P < 0.05.
Quantitative immunohistochemistry of MyoD-expressing cells was used to determine activated, satellite cell concentrations because MyoD is expressed following satellite cell activation (Cornelison & Wold, 1997). The concentration of MyoD-expressing cells in anti-F4/80-treated soleus muscles did not differ significantly from the number of MyoD-expressing cells in isotype control IgG-treated animals at 2 days of reloading (Fig. 5B). Isotype control IgG-treated animals showed a significant, 51% reduction in the concentration of MyoD-expressing cells (906 ± 105 cells mm−3, n = 5) compared to control IgG-injected mice at 2 days of reloading. In contrast, no change in the concentration of MyoD-expressing cells in the soleus muscles of anti-F4/80-treated cells occurred between days 2 and 4 of reloading (1993 ± 172 cells mm−3, n = 5) (Fig. 5B). Although previous investigators have noted that nuclei that were anti-MyoD immunoreactive could be located within muscle fibres in denervated muscle (Hyatt et al. 2003), we did not observe any MyoD-expressing nuclei within muscle fibres in our investigation.
Macrophage depletion between day 2 and day 4 of muscle reloading reduces muscle growth
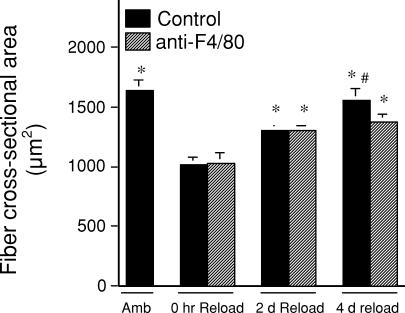
Changes in soleus muscle fibre cross-sectional area were used as an index of muscle atrophy or growth during muscle unloading and reloading. Similar to previous reports on rodents experiencing hindlimb muscle unloading, our results on animals receiving isotype antibody control injections showed 38% atrophy during 10 days of muscle unloading, followed by rapid recovery of size during reloading (return to 80% of original fibre cross-sectional area by 2 days of reloading and 95% of original cross-sectional area by 4 days of reloading). For much of the experimental time course the results were identical for anti-F4/80-treated mice. There was a 37% reduction in fibre cross-sectional area after 10 days of reloading, which returned to 79% of the original cross-sectional area after 2 days of reloading (Fig. 6). However, there was no further, significant increase in soleus fibre diameter in the anti-F4/80-treated mice between 2 days and 4 days of reloading.
Figure 6. Muscle fibres in the soleus muscle from mice treated with isotype control antibody and from mice treated with anti-F4/80.
Muscle fibres in the soleus muscle of mice treated with isotype control antibody increased in cross-sectional area during the interval between 0 and 4 days of reloading. However, fibres in muscles from mice treated with anti-F4/80 do not grow in cross-section during the interval between 2 and 4 days of reloading. *Significantly different from treatment-matched sample in 0 h reload group at P < 0.05; #significantly different from treatment-matched 2 day sample at P < 0.05.
Discussion
Previous investigations conducted in vitro have shown that macrophages are able to lyse muscle cells (Wehling et al. 2001; Nguyen & Tidball, 2003a), increase the rate of proliferation of myogenic cells (Robertson et al. 1993; Cantini & Carraro, 1995; Massimino et al. 1997; Chazaud et al. 2003), increase muscle cell survival (Chazaud et al. 2003), possibly promote satellite cell activation or muscle cell differentiation (Cantini & Carraro, 1995; Massimino et al. 1997) and decrease caspase-3 activity in myoblasts and myotubes, which may reflect a protective effect against apoptosis (Chazaud et al. 2003). In vivo observations have similarly supported potentially conflicting roles of macrophages in modulating muscle injury and repair. On one hand, depletion of macrophages from dystrophic mdx mice caused significant reductions in muscle membrane lysis and muscle damage (Wehling et al. 2001). However, administration of macrophage-conditioned media to muscles subjected to partial surgical ablation caused a more rapid increase in muscle mass during repair, than occurred in untreated, ablated muscle (Cantini et al. 2002), showing that a macrophage-derived product could have an anabolic effect on muscle following injury.
Other studies of injured muscle may indicate a role for macrophages in promoting muscle growth or repair in vivo. An earlier and thought-provoking investigation showed that expression of a lacZ reporter transgene under the control of the muscle-specific, desmin promoter was reduced in muscle fragments that were transplanted into a recipient mouse that had experienced whole-body irradiation, compared to a non-irradiated recipient (Lescaudron et al. 1999). Transgene expression in the non-irradiated recipient corresponded to the time and location of invasion of the transplant by CD11b-expressing leucocytes. Because macrophages can express CD11b, the observation suggested that macrophages may play a significant role in muscle repair or regeneration. More recently, null mutation of COX-2 or COX-2 inhibition was shown to cause a reduction in muscle fibre size in areas of muscle repair following injury, and decreases in MyoD mRNA in muscle (Bondesen et al. 2004). Because COX-2 inhibition or null mutation also reduced the numbers of CD11b-positive cells at sites of muscle repair, these findings may also reflect a role for muscle macrophages in muscle growth and repair.
The findings of the present investigation show that macrophages can play an important role in affecting remodelling of muscle membrane damage during modified muscle use. However, the present investigation and previous work (Tidball et al. 1999) provide no evidence for macrophage-mediated damage to muscle cell membranes during muscle reloading after periods of unloading. As the data in the present investigation show, membrane lesions that were indicated by procion orange dye influx were normally repaired between 2 and 4 days of muscle reloading, but the specific depletions of late-invading populations of macrophages prevented this repair. The elevated expression of dysferlin in muscle that is undergoing atrophy or in which there is increased muscle membrane damage indicates that membrane remodelling during modified muscle use may be mediated by dysferlin. Deficiency in dysferlin underlies the pathology of limb-girdle muscular dystrophy 2B (LGMD2B) and Miyoshi myopathy (Liu et al. 1998; Bashir et al. 1998), which are progressive muscle wasting diseases. Patients with LGMD2B and Miyoshi myopathy experience elevated muscle creatine kinase in the serum, suggesting that muscle membrane damage is a feature of the pathologies (Prelle et al. 2003). However, current evidence indicates that dysferlin plays a role in membrane repair, not membrane stability, because dysferlin-deficient mice show no increased membrane damage with increased muscle loading (Bansal et al. 2003). Instead, dysferlin-deficient mice show an accumulation of membrane vesicles subjacent to the cell membrane (Cenacchi et al. 2005), and more slowly repair membrane damage caused by injuring myotubes with a scalpel in vitro (Lennon et al. 2003) or intense irradiation of isolated fibres (Bansal et al. 2003). The current findings show that elevated dysferlin levels persist in muscle fibres during periods of muscle membrane damage caused by modified muscle use, and that membrane repair and normalization of dysferlin levels is positively influenced by macrophages.
The failure of macrophage-depleted muscles to regenerate or grow during days 2–4 of muscle reloading could potentially be caused by a defect in membrane repair that may be a prerequisite for growth or regeneration. However, previous studies using the same model of modified muscle use have shown that null mutation of gp91phox prevented muscle membrane lysis during reloading, but did not affect muscle fibre growth during reloading (Nguyen & Tidball, 2003c). This observation indicated that membrane damage is not required for subsequent fibre growth, suggesting that the mechanism through which macrophages promote muscle fibre growth during muscle reloading does not involve a role in membrane repair.
An alternative explanation for the negative effect of macrophage depletion on fibre growth or regeneration would be that depleted muscles may experience defects in satellite cell activation leading to impaired growth and regeneration during increased loading. This latter alternative has been supported by findings which showed that macrophages increase muscle cell proliferation and MyoD expression in vitro (Massimino et al. 1997), although the reductions of phagocytic macrophages in vivo did not cause a significant change in the number of MyoD-expressing cells in muscle following injury (Summan et al. 2006). However, our results are not completely consistent with these previous findings and the differences in the findings may be attributable to differences in the model systems studied or the phenotype of the macrophages that mediate the response. At 2 days of reloading when there was no depletion of macrophages in our treatment, we observed no significant difference in the concentration of MyoD-positive cells in muscles from anti-F4/80-treated or control mice. At 4 days of reloading, the number of MyoD-positive cells in the control muscle decreased significantly, although total satellite cell numbers (indicated by Pax7 labelling) were unchanged. This indicates that there was no further net increase of satellite cells between days 2 and 4 of reloading and that MyoD-positive cells may have differentiated to MyoD-negative cells during this interval. Differentiation beyond the stage at which MyoD was expressed would render the cells fusion-competent and able to contribute to fibre growth (Smith et al. 1994; Friday et al. 2003). However, there was no change in the numbers of MyoD-positive cells in macrophage-depleted muscles between 2 and 4 days of reloading, which may reflect their failure to differentiate so that fusion with fibres and fibre growth could occur.
The mechanisms that regulate the accumulation of two distinct populations of macrophages at different stages of muscle reloading are not known, although potential mechanisms have been suggested in previous investigations. Although early studies proved that a large proportion of leucocytes that accumulate in injured muscle invade from the vasculature in response to muscle injury (Bintliff & Walker, 1960), there is a small population of resident macrophages in healthy muscle that is phenotypically similar to the non-phagocytic subpopulation examined in the present investigation (Honda et al. 1990). Thus, part of the accumulation of the non-phagocytic macrophages may reflect proliferation of resident macrophages rather than chemoattraction from the vasculature. However, our previous experience has shown us that antibody depletion of resident populations of cells within the muscle parenchyma is very inefficient, and we anticipate that the reduction of non-phagocytic macrophages in reloaded muscle is a consequence of depletion of a precursor population of monocytes in circulation. Other observations show that the mechanisms that increase the numbers of phagocytic and non-phagocytic macrophages in muscle differ. Inhibition of complement activation by intraperitoneal injections of soluble complement receptor sCR1 significantly reduced the concentration of early invading, phagocytic macrophages in muscle but caused a small, transient increase in the numbers of non-phagocytic macrophages in a rat model of hindlimb suspension (Frenette et al. 2000). More recently, studies have also shown that activated satellite cells release factors that are chemoattractive to monocytes and macrophages in vitro (Chazaud et al. 2003). Nearly 80% of the chemoattraction of monocytes/macrophages to activated satellite cells was blocked by neutralizing antibodies to monocyte chemoattractant protein-1 (MCP-1), macrophage-derived chemokine, fractalkine, vascular endothelial growth factor, urokinase type plasminogen-activator receptor (μPAR) and urokinase. Because injured muscle tissue expresses elevated levels of mRNA for MCP-1 receptor and μPAR-1 (Barash et al. 2004), the chemokines that bind these receptors may be particularly likely candidates for chemoattraction to injured muscle. Although there are no data to address whether these chemokines selectively attract phagocytic or non-phagocytic macrophages, the observation that μPA-null mice have less leucocyte invasion into injured muscle that accompanies defects in muscle regeneration (Lluis et al. 2001) could feasibly reflect a defect in μPA chemoattraction of non-phagocytic macrophages.
Our findings suggest that macrophages that invade muscle 2–4 days following increased muscle use or injury can contribute to muscle repair, growth and regeneration by affecting muscle fibre membrane repair and satellite cell differentiation. These results are consistent with the model in which the macrophage subpopulation that first invades injured muscle serves to remove cellular debris, after which the subsequent invasive population participates in repair, regeneration and growth. This model has received other experimental support in a recent investigation in which the early invading, phagocytic macrophages were depleted prior to muscle injury by intravenous injections of liposomes that contained clodronate (Summan et al. 2006). Phagocytic cells such as macrophages internalize and degrade the liposomes, thereby releasing the clodronate that induces apoptosis. Clodronate treatment produced a large, significant reduction in muscle macrophages at 3 days after injury. However, the depletion of macrophages at this time point caused an increase in muscle macrophages at 9 days after injury. Unlike the findings of the present investigation in which the late-invading macrophages were targeted, depletion of the early invading population produced a slowing of the removal of cellular debris, but had no effect on muscle regeneration, on the effect of muscle fibre growth following injury, or on satellite cell differentiation that was reflected as levels of MyoD expression. Thus, in vivo observations now provide functional data for two populations of macrophages involved in distinct roles in the response of muscle to injury. These findings highlight the potential value of targeting macrophages for modulating the repair and growth of skeletal muscle, but also emphasize the likely importance of selectively manipulating the numbers or activities of specific subpopulations of macrophages for designing therapeutic strategies.
Acknowledgments
This investigation was supported by grants from the National Institutes of Health (AR47721 and AR47855) and the Muscular Dystrophy Association, USA. We are grateful to Katherine Wen and Anna Avik for valuable technical contributions to the investigation. The Pax-7 monoclonal antibody developed by Dr Atsushi Kawakami was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute for Child Health and Development and maintained by the University of Iowa, Iowa City, IA, USA.
References
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Amer J Physiol Cell Physiol. 2004;286:C355–C364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- Bigard AX, Merino D, Leinhard F, Serrurier B, Guezennec CY. Quantitative assessment of degenerative changes in soleus muscle after hindlimb suspension and recovery. Eur J Appl Physiol Occup Physiol. 1975;75:380–387. doi: 10.1007/s004210050176. [DOI] [PubMed] [Google Scholar]
- Bintliff S, Walker BE. Radioautographic study of skeletal muscle regeneration. Am J Anat. 1960;106:233–245. [Google Scholar]
- Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Amer J Physiol Cell Physiol. 2004;287:C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- Burnette WN. ‘Western blotting’: electrophoretic transfer of proteins from sodium dodecyl sulfate–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol. 1995;54:121–128. doi: 10.1097/00005072-199501000-00014. [DOI] [PubMed] [Google Scholar]
- Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vittiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci. 2002;23:189–194. doi: 10.1007/s100720200060. [DOI] [PubMed] [Google Scholar]
- Cenacchi G, Fanin M, De Giorgi LB, Angelini C. Ultrastructural changes in dysferlinopathy support defective membrane repair mechanism. J Clin Pathol. 2005;58:190–195. doi: 10.1136/jcp.2004.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol A-C, Poron F, Authier F-J, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopard A, Arrighi N, Carnino A, Marini JF. Changes in dysferlin, proteins from dystrophin glycoprotein complex, costameres, and cytoskeleton in human soleus and vastus lateralis muscles after a long-term bedrest with or without exercise. FASEB J. 2005;19:1722–1724. doi: 10.1096/fj.04-3336fje. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Frenette J, Cai B, Tidball JG. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol. 2000;156:2103–2110. doi: 10.1016/S0002-9440(10)65081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday BB, Mitchell PO, Kegley KM, Pavlath GK. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–227. doi: 10.1046/j.1432-0436.2003.710303.x. [DOI] [PubMed] [Google Scholar]
- Honda H, Kimura H, Rostami A. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology. 1990;70:272–277. [PMC free article] [PubMed] [Google Scholar]
- Hyatt JP, Roy RR, Baldwin KM, Edgerton VR. Nerve activity-independent regulation of skeletal muscle atrophy: role of MyoD and myogenin in satellite cells and myonuclei. Am J Physiol Cell Physiol. 2003;285:C1161–C1173. doi: 10.1152/ajpcell.00128.2003. [DOI] [PubMed] [Google Scholar]
- Kasper CE. Sarcolemmal disruption in reloaded atrophic skeletal muscle. J Appl Physiol. 1995;79:607–614. doi: 10.1152/jappl.1995.79.2.607. [DOI] [PubMed] [Google Scholar]
- Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993;16:99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Peltekain E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- Lluis F, Roma J, Suelves M, Parra M, Aniorte G, Gallardo E, Illa I, Rodriguez L, Hughes SM, Carmeliet P, Roig M, Muñoz-Canovez P. Urokinase-dependent plasminogen activation is required for efficient skeletal muscle regeneration in vivo. Blood. 2001;97:1703–1711. doi: 10.1182/blood.v97.6.1703. [DOI] [PubMed] [Google Scholar]
- McLennan IS. Resident macrophages (ED2- and ED3-positive) do not phagocytose degenerating rat skeletal muscle fibers. Cell Tissue Res. 1993;272:193–196. doi: 10.1007/BF00323586. [DOI] [PubMed] [Google Scholar]
- Massimino M, Rapizzi E, Cantini M, Libera L, Mazzoeni F, Arsian P, Carraro U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun. 1997;235:754–759. doi: 10.1006/bbrc.1997.6823. [DOI] [PubMed] [Google Scholar]
- Minamide LS, Bamburg JR. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal Biochem. 1990;190:66–70. doi: 10.1016/0003-2697(90)90134-u. [DOI] [PubMed] [Google Scholar]
- Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- Nguyen HX, Tidball JG. Interactions between neutrophils and macrophages promote macrophage killing of muscle cells in vitro. J Physiol. 2003a;547:125–132. doi: 10.1113/jphysiol.2002.031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, Tidball JG. Expression of a muscle-specific, nitric oxide synthase transgene prevents muscle membrane injury and reduces muscle inflammation during modified muscle use. J Physiol. 2003b;550:347–356. doi: 10.1113/jphysiol.2003.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, Tidball JG. Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J Physiol. 2003c;553:833–841. doi: 10.1113/jphysiol.2003.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelle A, Sciacco M, Tancredi L, Fagiolari G, Comi GP, Ciscato P, et al. Clinical, morphological and immunological evaluation of six patients with dysferlin deficiency. Acta Neuropathol (Berl) 2003;105:537–542. doi: 10.1007/s00401-002-0654-1. [DOI] [PubMed] [Google Scholar]
- Robertson TA, Maley MA, Grounds MD, Papadimitriou JM. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res. 1993;207:321–331. doi: 10.1006/excr.1993.1199. [DOI] [PubMed] [Google Scholar]
- St Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading following rat hindlimb suspension. J Appl Physiol. 1994;77:290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- Seale P, Rudnicki MA. A new look at the origin, function, and ‘stem-cell’ status of muscle satellite cells. Dev Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- Smith CK, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol. 1994;159:379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4+) and cytotoxic (CD8+) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, Simeonova PP. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1448–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol. 1999;65:492–498. [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–132. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]