Abstract
The pelvic autonomic nervous system is a target for circulating androgens in adults, with androgen exposure or deprivation affecting the structure and function of urogenital tract innervation. However, the critical period for androgen exposure to initially establish pelvic autonomic neuromuscular transmission has not been determined. We have examined the sympathetic innervation of the vas deferens in hypogonadal (hpg) mice that are deprived of androgens after birth but undergo normal prenatal sexual differentiation and remain androgen responsive throughout life. In vasa deferentia from hpg mice, purinergic excitatory junction potentials and contractions could not be elicited by electrical stimulation and P2X1 purinoceptors could not be demonstrated by immunofluorescence. Moreover, a novel inhibitory nitrergic transmission developed. Administering testosterone to adult hpg mice restored purinergic excitatory transmission and P2X1 purinoceptor immunofluorescence, and nitrergic inhibitory transmission was lost. Despite the deficit in excitatory neurotransmission in hpg mice, their vasa deferentia were innervated by numerous noradrenergic axons and pelvic ganglia appeared normal. In addition, noradrenergic contractions could be elicited by electrical stimulation. This study has revealed that postnatal androgen exposure has a profound effect on the development of excitatory transmission in vas deferens smooth muscle, primarily by a postjunctional action, but is not essential for development of the structural innervation of this organ. Our results also indicate that there is no postnatal critical period for androgen exposure to establish neuroeffector transmission and that postnatal androgen exposure can be delayed until adulthood, with little consequence for establishment of normal sympathetic neurotransmission.
Gonadal steroids are important for the establishment of sexual dimorphism in the nervous system (Simerly, 2002; Morris et al. 2004). Many of these effects are permanent and, beyond a critical period, can no longer be altered by steroid exposure or deprivation. Androgens and oestrogens continue to exert powerful but transitory (reversible) actions on adult neurons, which have been best documented in areas of the central nervous system directing reproductive behaviour (McEwen, 2001; Becker et al. 2005; Cooke & Woolley, 2005). Pelvic ganglia comprise the link between central controls and organ function, as they provide all of the motor innervation of the reproductive organs, and therefore the activity of these neurons is critical for successful mating. Androgens regulate many features of adult pelvic autonomic neurons, including soma size, terminal field, and transmitter expression (reviewed by Keast, 2006), deprivation leading to deficits in penile erection and neurotransmission to smooth muscle of the male reproductive organs. The role of androgens in early development to establish a functional nerve supply in these organs is not known. A limited number of studies in rats have suggested that for some aspects of neuronal chemistry and structure there is a critical period for androgen exposure within the first two postnatal weeks (Hamill & Guernsey, 1983; Melvin & Hamill, 1986); however, these studies did not assess neuronal function.
To define if and how androgens are required to initially establish neuromuscular transmission in the urogenital tract, we investigated the sympathetic innervation of the vas deferens, which is essential for propulsion of sperm and seminal fluid during copulation. The physiological, pharmacological and anatomical features of this nerve supply have been extensively studied. Possible defects in the projections from pelvic ganglia to the vas deferens were assessed in adult hypogonadal (hpg) mice, which are deprived of androgens after birth, using in vitro recording of neuromuscular transmission and immunohistochemistry. To determine whether there is a postnatal critical period for androgen exposure, i.e. whether any deficits in adult hpg mice could not be reversed, some adult hpg animals were treated with testosterone.
We hypothesized that the development of vas deferens innervation would be severely impeded by the prolonged lack of androgens in adult hpg mice and that delayed exposure to androgens would not reverse the effects. To our surprise, in the adult hpg mice androgen deprivation affected excitatory neuroeffector transmission but not the structure of the sympathetic nerves and a completely novel type of inhibitory neurotransmission developed. Moreover, administering testosterone to adult hpg mice reversed all effects of androgen deprivation on neuromuscular transmission.
Methods
Animal production, anaesthesia and tissue removal
All procedures were approved by the Animal Care and Ethics Committees of the University of Sydney, University of New South Wales and Animal Welfare Committee of Sydney South West (formerly Central Sydney) Area Health Service, in compliance with guidelines of the National Health and Medical Research Council of Australia. Every effort was made to avoid animal suffering and to minimize the numbers of animals. All animals were housed under standard 12 h: 12 h lighting conditions and had free access to chow and water.
Male hpg mice were bred as previously described (Singh et al. 1995). All mice were studied between 8 and 16 weeks of age (11.5 ± 1.9 weeks; mean ±s.d.). Homozygous (N/N) normal and heterozygous (N/hpg) mice of this strain served as controls. The hpg mutation is autosomal recessive and heterozygous animals undergo identical sexual differentiation to wild-types (Cattanach et al. 1977). In some hpg mice an implant made of Silastic tubing filled with crystalline testosterone (Sigma, Castle Hill, NSW, Australia) was inserted beneath the dorsal skin under anaesthesia (20 mg kg−1 xylazine, 80 mg kg−1 ketamine i.p.) at 6–10 weeks of age (7.8 ± 1.2 weeks, range 5.5–10.2 weeks) (Singh & Handelsman, 1999); this was a very brief procedure and there was no need for supplemental anaesthetic doses. These mice were studied at 4–8 weeks post-implantation (4.9 ± 1.3 weeks, range 3.5–7.7 weeks) and have been referred to as hpgT. Tissue removal for pharmacological studies was performed following cervical dislocation. For immunohistochemical studies, tissues were either removed following cervical dislocation and then immersed in fixative overnight (4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4) or removed from mice which were anaesthetized (20 mg kg−1 xylazine, 80 mg kg−1 ketamine i.p.) and then perfused intracardially with the same fixative, followed by overnight post-fixation in the same solution. There was no difference in the immunohistochemical results obtained between these two types of fixation. Fixed tissues were washed with phosphate-buffered saline (PBS; 0.1 m, pH 7.4) and stored in PBS containing 0.1% sodium azide.
Electrophysiological studies
The whole vas deferens was pinned to the Sylgard coated base of a 1 ml recording chamber that was continuously perfused with physiological saline (3–5 ml min−1) of the following composition (mm): NaCl 133.5; NaHCO3 16.3; d-glucose 7.8; KCl 4.7; CaCl2 2.0; NaH2PO4 1.3; MgCl2 1.2. This solution was heated to 37°C and was bubbled with 5% CO2–95% O2 (pH 7.25). The prostatic end of the vas deferens was drawn into a suction electrode and the nerves excited by electrical stimuli (3–10 V, 1 ms pulse width). At the beginning of each experiment the supramaximal voltage for activating the nerves was determined (typically 5 V). Intracellular recordings were made from the mid section of the vas deferens using glass microelectrodes (120–200 MΩ) filled with 0.5 m KCl and connected to an Axoclamp bridge amplifier (Axon Instruments, Inc., Union City, CA, U.S.A). Membrane potentials were determined upon withdrawal of the microelectrode.
Mechanical studies
To assess the contractile properties of the longitudinal smooth muscle of the vas deferens, the whole tissue was dissected out and suspended vertically in a 10 ml organ bath. The prostatic end of tissue was attached to a tissue holder and the epididymal end was attached to an isometric force transducer (Grass FT03). Electrical field stimuli (20–50 V, 1 ms pulse width) were applied through a pair of platinum ring electrodes (diameter 2 mm, separation 10 mm) mounted around the vas deferens. In a separate series of experiments, the contractile properties of the circular smooth muscle of the vas deferens were also assessed. In these experiments ∼2 mm segments from the middle of the vas deferens were mounted isometrically between stainless steel wires (50 μm diameter) in a four-chamber myograph (Multi Myograph Model 610M, Danish Myo Technology, Denmark) and electrical field stimuli (10–25 V, 0.2 ms pulse width) were applied through platinum plate electrodes mounted on either side of the tissue along its length. All tissues were superfused with physiological saline (see above) and maintained at 37°C.
In both sets of experiments, initially control and hpgT tissues had a tension of ∼10 mN applied whereas hpg tissues, which were smaller, had a tension of ∼5 mN applied. After applying tension, the tissues were allowed to equilibrate for 30 min. At the start of all experiments a supramaximal stimulation voltage for evoking contraction was determined (typically 40 V for the longitudinal muscle and 15 V for the circular muscle) and it was confirmed that at this voltage the contractions were blocked by tetrodotoxin (TTX, 0.5 μm). The hpg vasa deferentia were much smaller than those from control mice, but we did not correct the contractions for this difference in tissue size. Apart from rauwolscine, all drugs were supplied by Sigma (Castle Hill, NSW, Australia). Rauwolscine was supplied by Tocris Cookson Ltd (Bristol, UK). In the experiments where the K+ concentration was raised to 60 mm, the physiological saline (see above) had an equimolar substitution of KCl for NaCl and tissues were pretreated with prazosin (0.1 μm) and suramin (0.1 mm) to prevent the possible postjunctional actions of noradrenaline and ATP released from the nerve terminals by K+-evoked depolarization.
The electrophysiological data and tissue weights are presented as the mean ± 1 s.d. and all other measures presented as the mean ± 1 s.e.m. For multiple comparisons one-way analysis of variance and the Games–Howell post hoc test were used. Other pairwise comparisons were made with Student's t test for paired or unpaired data.
Immunohistochemistry
Immunohistochemistry was performed on vasa deferentia from four to six mice per group (control, hpg, hpgT). Vasa deferentia were cryoprotected in PBS containing 30% sucrose prior to cutting longitudinal or transverse cryosections (14 μm). From each vas deferens, at least eight non-consecutive sections were immunostained for each substance of interest. Sections were processed for immunofluorescence as previously described (Wanigasekara et al. 2004). Briefly, sections were incubated for 1 h in blocking solution (PBS containing 10% non-immune horse serum and 0.1% triton X-100), then for 18–24 h in primary antisera, at room temperature (Table 1A). The following day unbound primary antiserum was removed by washing and tissues incubated for 2–3 h in secondary antisera at room temperature. Following a further wash, sections were coverslipped using bicarbonate-buffered glycerol (0.5 m, pH 8.6) and viewed under an Olympus BX-51 microscope.
Table 1.
Antisera used for immunohistochemical studies
| A. Primary antisera | ||||
|---|---|---|---|---|
| Antigen | Abbreviation | Host | Dilution | Supplier |
| Calcitonin gene-related peptide | CGRP | Goat | 1: 1000 | Biogenesis (Poole, UK) |
| Dopamine-β-hydroxylase | DBH | Rabbit | 1: 5000 | Diasorin (Stillwater, MN, USA) |
| Neuropeptide Y | NPY | Sheep | 1: 1000 | Auspep (Melbourne, Australia) |
| Nitric oxide synthase | NOS | Rabbit | 1: 500 | Zymed (San Francisco, CA, USA) |
| Nitric oxide synthase | NOS | Sheep | 1: 5000 | Prof P Emson (Babraham, UK) |
| P2X1 purinoceptor | P2X1 | Rabbit | 1: 2000 | Alomone Laboratories (Jerusalem, Israel) |
| Synapsin | SAP | Rabbit | 1: 1000 | Biogenesis |
| Synaptophysin | SYN | Mouse | 1: 100 | Dako (Glostrup, Denmark) |
| Tyrosine hydroxylase | TH | Rabbit | 1: 500 | Chemicon (Temecula, CA, USA) |
| Tyrosine hydroxylase | TH | Sheep | 1: 1500 | Chemicon |
| Vasoactive intestinal peptide | VIP | Rabbit | 1: 2000 | Diasorin |
| Vesicular acetylcholine transporter | VAChT | Goat | 1: 3000 | Chemicon |
| B. Secondary antisera | |||
|---|---|---|---|
| Antigen | Host | Conjugate | Dilution |
| Goat | Donkey | Cy-3 | 1: 1000 |
| Mouse | Donkey | Cy-3 | 1: 1000 |
| Mouse | Donkey | Cy-2 | 1: 200 |
| Rabbit | Donkey | Cy-3 | 1: 1500 |
| Rabbit | Donkey | FITC | 1: 100 |
| Sheep | Donkey | FITC | 1: 200 |
All secondary antisera were obtained from Jackson Immunoresearch (West Grove, PA).
For direct visualization of catecholamines in cryosections of vas deferens we used the sucrose–phosphate–glyoxylic acid method (de la Torre, 1980). Briefly, cryosections were dipped in glyoxylic acid solution, air-dried for 20 min, heated for 6 min at 95°C, coverslipped with Depex and viewed under the fluorescence microscope.
Pelvic ganglia were processed for immunofluorescence as whole mounts, costaining for tyrosine hydroxylase (TH) and nitric oxide synthase (NOS), markers of noradrenergic (sympathetic) and cholinergic (parasympathetic) neurons, respectively (Wanigasekara et al. 2004) (Table 1A). This closely resembled the cryosection staining procedure except the blocking solution contained 0.5% Triton X-100 and some incubation times were longer (18–24 h for blocking solution, 4–6 h or overnight for secondary antisera). Neurons were counted manually, focusing throughout the thickness of each ganglion.
Images were obtained using a Spot RT-Slider cooled CCD camera (Diagnostic Instruments, Sterling Heights, MI, USA). Figures were produced using Adobe Illustrator (version 8), adjusting contrast and brightness where necessary to best represent the immunostaining viewed under the microscope using Adobe Photoshop (version 8). For quantification of P2X1 purinoceptor expression, immunofluorescence intensity was quantified using ImageJ software (public domain software; rsb.info.nih.gov/ij/). Images were acquired under the 40× objective, using the same exposure for each section and each animal. An area of 400 × 400 pixels was chosen randomly from four regions of smooth muscle from each of five animals per group; this box included approximately two-thirds of the thickness of the smooth muscle layer in each section. Mean fluorescence intensity was assessed by first thresholding the images so that all of the immunostained plasma membrane structures were identified; the mean fluorescence intensity of these structures was then quantified in each region. Tissues from all animal groups were immunostained in the same run.
Data are expressed as the mean ±s.e.m. and comparisons made using Student's t test for unpaired data (P2X1, control versus hpgT) or one-way ANOVA (number of ganglion cells in each animal group). For all statistical tests, P-values < 0.05 were taken to indicate a significant difference.
Results
Following prolonged androgen deprivation, excitatory junction potentials were absent and a novel nitrergic inhibitory junction potential appeared
In vasa deferentia from control mice (n = 3), each electrical stimulus evoked a transient depolarization with a duration < 300 ms. In 13 of 16 cells studied, these excitatory junction potentials (EJPs) had an amplitude of 34 ± 6 mV (mean ±s.d.) and did not vary greatly in size from impulse to impulse (Fig. 1A). Despite their large amplitude, these EJPs did not evoke muscle action potentials. In these cells the resting membrane potential (RMP) was −73 ± 6 mV. In the three remaining cells, EJPs varied markedly in amplitude and the larger amplitude EJPs triggered muscle action potentials (Fig. 1C). The RMP for these three cells was −63 ± 2 mV. Cells displaying these two types of activity have previously been described in the mouse vas deferens (Holman et al. 1977). In all cells spontaneous EJPs were recorded (Fig. 1A and B).
Figure 1. Intracellularly recorded electrical activity in control and hpgT vasa deferentia.
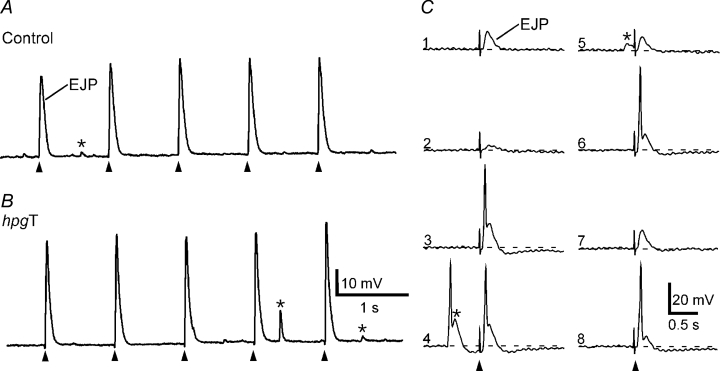
A and B, EJPs evoked by 5 stimuli at 1 Hz in a control (A) and an hpgT (B) vas deferens. C, representative traces from a cell in a control vas deferens in which EJPs evoked during trains at 1 Hz were more variable in amplitude (numbers indicate sequence of stimuli) and occasionally evoked muscle action potentials. Cells displaying this pattern of activity were also recorded in hpgT vas deferens. In both control and hpgT vasa deferentia, spontaneous EJPs were recorded (indicated by *). In trace 4 of panel C, a muscle action potential was triggered by a spontaneous EJP. In A, B and C, the resting membrane potentials were −76, −79 and −61 mV, respectively.
The vasa of hpg mice were substantially smaller than those of the controls (wet weight: control 14.4 ± 2.4 mg, mean ±s.d., n = 6; hpg 1.3 ± 0.3 mg, n = 5), as reported previously for other male reproductive organs in these mice (Bianco et al. 2002). In the 23 cells studied in vasa deferentia from hpg mice (n = 5), electrical stimulation did not evoke EJPs. Instead, in all cells, electrical stimulation produced hyperpolarization of the smooth muscle membrane (Fig. 2A). In response to single stimuli a small amplitude (−2 ± 1 mV) inhibitory junction potential (IJP) with an overall duration > 30 s was recorded (Fig. 2A). Trains of stimuli at 0.03–0.5 Hz evoked larger amplitude IJPs (Fig. 2A). In these cells the RMP was −56 ± 5 mV. IJPs were blocked by l-NAME (0.1 mm, n = 3, Fig. 2B). Spontaneous EJPs were not recorded in hpg vasa deferentia.
Figure 2. Intracellularly recorded electrical activity recorded in hpg vasa deferentia.
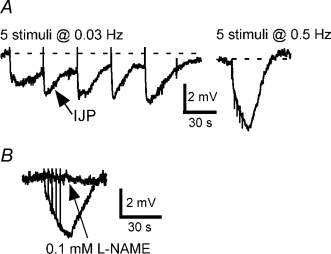
A, inhibitory junction potentials (IJPs) evoked by 5 stimuli at 0.03 and 0.5 Hz. B, overlaid traces showing responses to 5 stimuli at 0.5 Hz before and during application of l-NAME (0.1 mm) in another tissue. In A and B, the resting membrane potentials were −58 and −52 mV, respectively.
Postnatal androgen deprivation severely impeded neurally evoked mechanical responses
Activation of either the longitudinal (n = 6) or the circular (n = 6) muscle of vasa deferentia from control mice with trains of 20 pulses at 10 Hz evoked transient contractions that peaked and then declined in force during the period of stimulation (Fig. 3A). In the longitudinal muscle of vasa deferentia from one of the six hpg mice studied, stimulation with trains at 50 V failed to evoke contraction. For the longitudinal muscle of the other five tissues studied, electrical stimulation evoked contractions that were much smaller than those of the control tissues (Fig. 3A and B). Similarly, in comparison with control tissues, electrical stimulation of the circular muscle of hpg mice (n = 6) evoked very small contractions (Fig. 3A and B).
Figure 3. Neurally evoked mechanical responses of control, hpg and hpgT vasa deferentia.
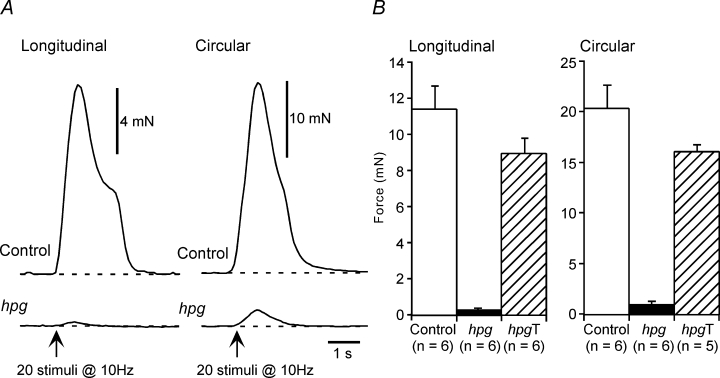
A, representative traces showing contractions of the longitudinal and circular smooth muscle of control and hpg vasa deferentia to 20 stimuli at 10 Hz. B, bar graphs showing the contraction amplitude data for the longitudinal and circular smooth muscle of vasa deferentia from control, hpg and hpgT mice.
Small amplitude (< 0.2 mN) spontaneous contractions were observed in the longitudinal muscle of vasa deferentia from 4 of the 10 hpg mice studied. Such spontaneous activity was not observed in the longitudinal muscle of control tissues or in the circular muscle of either control or hpg tissues.
Postnatal androgen deprivation selectively abolished the purinergic component of contractions evoked by electrical stimulation
The effects of neurotransmitter antagonists on electrically evoked contractions were assessed in the circular muscle. In control vasa deferentia (n = 6), the α1-adrenoceptor antagonist prazosin (0.1 μm) and the P2-purinoceptor antagonist suramin (0.1 mm) when applied individually reduced the amplitude of the contractions (Fig. 4A–C) and in combination these agents almost abolished the contractions (reduced by 91 ± 1%). In contrast, in hpg vasa deferentia (n = 6), prazosin almost abolished electrically evoked contractions whereas suramin significantly increased the amplitude of the contractions (Fig. 4A–C). In addition, in hpg vasa deferentia, there were no differences in the blockade produced by prazosin alone (reduced by 93 ± 6%) or by the combination of prazosin and suramin (reduced by 92 ± 5%, paired t test P = 0.13).
Figure 4. Neurally evoked contractions of control and hpgT vasa deferentia were reduced by either prazosin or suramin but those of hpg vasa deferentia were only reduced by prazosin.
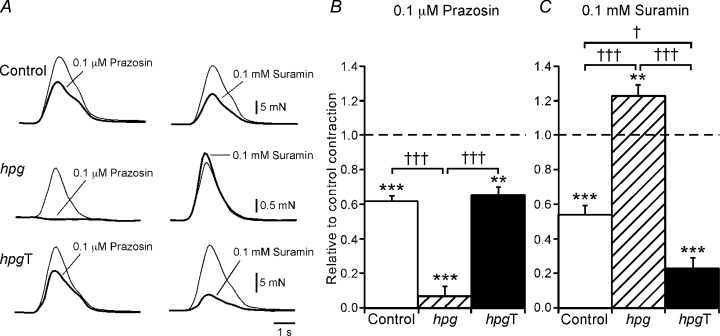
A, overlaid traces showing contractions of the circular smooth muscle of control (upper traces), hpg (middle traces) and hpgT (lower traces) vasa deferentia evoked by 20 stimuli at 10 Hz under control conditions (thin line) and in the presence of either prazosin (0.1 μm) or suramin (0.1 mm) (thick line). B and C, bar graphs showing the relative change in contraction of control, hpg and hpgT vasa deferentia produced by prazosin (B) and suramin (C). Comparisons with control recordings in the same tissues were made with paired t tests (**P < 0.01, ***P < 0.001). Comparisons between the groups of tissues were made with one-way ANOVA followed by Games–Howell tests (†P < 0.05, †††P < 0.001).
Blocking neuronal uptake of released noradrenaline with desmethylimipramine (DMI, 0.1 μm) increased the amplitude of electrically evoked contractions of the circular muscle of hpg vasa deferentia (increased by 73 ± 18%, n = 5, paired t test P < 0.05). In contrast, in control tissues (n = 6), DMI reduced the amplitude of electrically evoked contractions (reduced by 27 ± 4%, paired t test P < 0.01). In both groups of tissues, the subsequent application of the α2-adrenoceptor antagonist rauwolscine (0.1 μm) increased the amplitude of the contractions but this effect was more marked in the control tissues (increased by 148 ± 20%, n = 6, paired t test P < 0.001) than in the hpg tissues (increased 26 ± 5%, n = 5, paired t test P < 0.01). In the presence of both DMI and rauwolscine, the further addition of prazosin (0.1 μm) almost abolished contractions of hpg vas deferentia (reduced by 98 ± 1%) whereas those of control vasa deferentia were reduced (by 68 ± 4%).
In the circular muscle of hpg mice, l-NAME (0.1 mm, n = 5) produced an increase in the force of electrically evoked contractions (increased by 215 ± 67%, paired t test P < 0.05). This enhancement of electrically evoked contraction by l-NAME was not observed in the circular muscle of control vasa deferentia (increased by 1 ± 4%, n = 6, paired t test P = 0.85). In hpg vasa deferentia, application of atropine (1 μm, n = 4) produced a significant decrease in the force of electrically evoked contractions (reduced by 33 ± 6%, paired t test P < 0.01). As previously reported for mouse vas deferens (Cuprian et al. 2005), atropine did not significantly change the amplitude of neurally evoked contractions of control tissues (reduced by 11 ± 5%, n = 4, paired t test P = 0.14).
Postnatal androgen deprivation abolished the contractions to α,β-methylene ATP but not to phenylephrine
In the longitudinal muscle of control vasa deferentia (n = 6), the α1-adrenoceptor agonist phenylephrine (10 μm) produced contractions that peaked and then waned during the period of application (maximum amplitude 2.5 ± 0.9 mN). By contrast, in the longitudinal muscle of hpg vasa deferentia (n = 9) phenylephrine (10 μm) produced smaller amplitude oscillatory contractions (maximum amplitude 0.8 ± 0.2 mN). The circular muscle of control vasa deferentia (n = 6) was less responsive to phenylephrine, with 100 μm being required to produce contractions similar in peak amplitude to those evoked by 10 μm in the longitudinal muscle (Fig. 5A and D). The higher concentration of phenylephrine produced smaller amplitude contractions of the circular muscle in hpg vasa deferentia (Fig. 5A and D). The P2X-purinoceptor agonist α,β-methylene ATP (10 μm) produced a transient contraction in both the longitudinal (maximum amplitude 9.5 ± 1.2 mN, n = 4) and circular (n = 6; Fig. 5B and E) muscle of control vasa deferentia but had no contractile effect on either the longitudinal (n = 4) or the circular (n = 5; Fig. 5B and E) muscle of hpg vasa deferentia.
Figure 5. Mechanical responses of control, hpg and hpgT vasa deferentia to phenylephrine, α,β-methylene ATP and 60 mm K+.
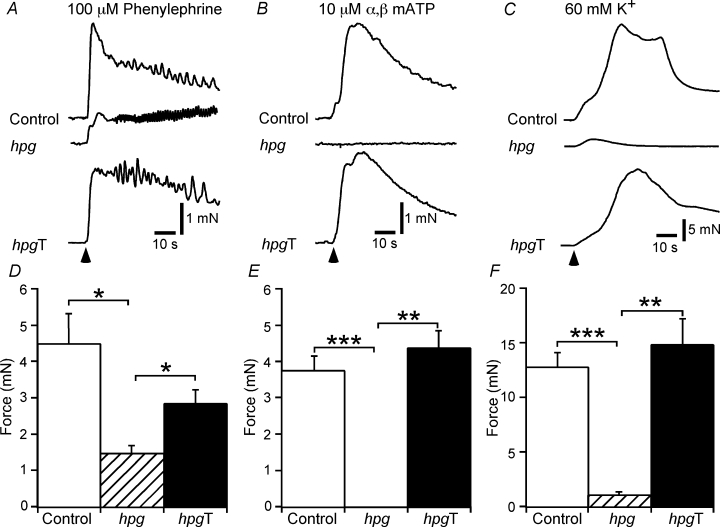
A–C, representative traces showing contractions of the circular smooth muscle of control, hpg and hpgT vasa deferentia to phenylephrine (100 μm, A), α,β-methylene ATP (10 μm, B) and 60 mm K+ (C). D–F, bar graphs showing the peak amplitude of contractions of control, hpg and hpgT vasa deferentia to phenylephrine, α,β-methylene ATP and 60 mm K+. Comparisons between the groups of tissues were made with one-way ANOVA followed by Games–Howell tests (*P < 0.05, **P < 0.01, ***P < 0.001).
The effects of raising the K+ concentration to 60 mm were only assessed in the circular muscle. In control and hpg vasa deferentia, this stimulus produced contractions that peaked and then waned during the period of application (Fig. 5C and D). However, in comparison with the responses of control vasa deferentia, those of hpg vasa deferentia were much smaller in amplitude and faster in time course (Fig. 5C and D).
Delayed replacement of testosterone reversed all of the effects of androgen deprivation on neurotransmission
Testosterone was administered to adult hpg animals to determine if the normal adult phenotype could be ‘rescued’. Vasa deferentia from hpgT mice were substantially larger than those of hpg mice (wet weight 10.8 ± 1.0 mg, mean ±s.d., n = 5) but they were lighter than those of control mice (see above, unpaired t test P < 0.05). Electrical activity recorded in vasa deferentia from hpgT mice (n = 5) was similar to that recorded in tissues from control mice. In each of the 38 cells studied, electrical stimulation evoked EJPs. In 19 of these cells, EJPs had large amplitudes (37 ± 7 mV, mean ±s.d.) and did not vary greatly in size during trains of stimuli (Fig. 1B). In these cells, EJPs did not trigger muscle action potentials and the RMP was −76 ± 4 mV. In the remaining 15 cells, EJPs fluctuated in amplitude. In these cells the larger amplitude EJPs triggered muscle action potentials and the RMP was −58 ± 8 mV. As in control tissues, spontaneous EJPs were recorded in all cells in hpgT vasa deferentia (Fig. 1B).
Electrically evoked contractions of the longitudinal (n = 6) and circular (n = 5) muscle of vasa deferentia from hpgT mice were similar to those of control mice (Fig. 3B). While the peak amplitude of these contractions tended to be smaller in hpgT vasa deferentia (Fig. 3B), this difference did not reach the level of statistical significance (unpaired t test, longitudinal muscle P = 0.11, circular muscle P = 0.13). The longitudinal muscle of all hpgT vasa deferentia generated spontaneous contractions (up to about 3 mN in amplitude). This activity was not affected by adrenoceptor or purinoceptor antagonists or by TTX. No spontaneous activity was observed in the circular muscle.
The blockade of the electrically evoked contractions of the circular muscle produced by prazosin (0.1 μm) did not differ between hpgT and control tissues (Fig. 4A and B, Games–Howell test P = 0.83) but that produced by suramin (0.1 mm) was greater for hpgT vasa deferentia (Fig. 4A and C). In combination, these agents reduced contractions of hpgT vasa deferentia by 95 ± 1% and this effect did not differ from that observed for control vasa deferentia (see above; Games–Howell test P = 0.13). Atropine (1 μm, n = 5) did not significantly change electrically evoked contractions of the circular muscle of hpgT vasa deferentia (reduced by 6 ± 6%, paired t test P = 0.43) but l-NAME (0.1 mm, n = 5) significantly reduced contractions of these tissues (reduced by 15 ± 4%, paired t test P < 0.05).
The peak amplitude of phenylephrine (10 μm)-induced contractions of the longitudinal muscle of hpgT vasa deferentia (2.5 ± 1.2 mN, n = 7) did not differ from that observed in control tissues (see above, unpaired t test P = 0.93). Similarly, in the circular muscle, the peak amplitude of contractions to phenylephrine (100 μm) did not differ significantly between hpgT and control vasa deferentia (Fig. 5A and D, Games–Howell test P = 0.43). While the effects of α,β-methylene ATP (10 μm) were not studied in the longitudinal muscle of hpgT vasa deferentia, in the circular muscle the peak amplitude of contractions to this agent were similar to those observed in control tissues (Fig. 5B and E, Games–Howell test P = 0.60). The peak amplitude of contractions of the circular muscle evoked by raising the K+ concentration to 60 mm also did not differ between hpgT and control vasa deferentia (Fig. 5C and F, Games–Howell test P = 0.72).
Despite deficits in neurotransmission, noradrenergic and cholinergic innervation was retained after prolonged postnatal androgen deprivation
Although excitatory junction potentials could not be elicited in vasa deferentia from adult hpg mice, the muscle was supplied by a dense plexus of TH axons (Fig. 6, top row). In contrast, whereas in control mice NOS axons were largely restricted to the subepithelial plexus, in hpg mice the NOS axons also formed a dense plexus in the muscle (Fig. 6, bottom row). No further differences were identified when studies were performed with additional markers for these two nerve types. For example, in the smooth muscle of hpg vasa deferentia (Fig. 7B), numerous axons showed catecholamine histofluorescence, as also seen in control tissue (Fig. 7A). TH was coexpressed with dopamine-β-hydroxylase (DBH, Fig. 7C), synapsin (SAP, Fig. 7D), neuropeptide Y and synaptophysin (not shown). NOS-immunoreactive axons coexpressed vasoactive intestinal peptide (VIP, Fig. 7E) and vesicular acetylcholine transporter (VAChT; Fig. 7F). Sensory fibres labelled with calcitonin gene-related peptide (CGRP) were sparse (Fig. 7G). The vas deferens muscle from hpgT mice was supplied by numerous TH axons (Fig. 6, top row), but NOS-immunoreactive cholinergic axons were much sparser than in hpg mice (Fig. 6, bottom row).
Figure 6. Autonomic innervation of vasa deferentia in adult control, hpg and hpgT mice.
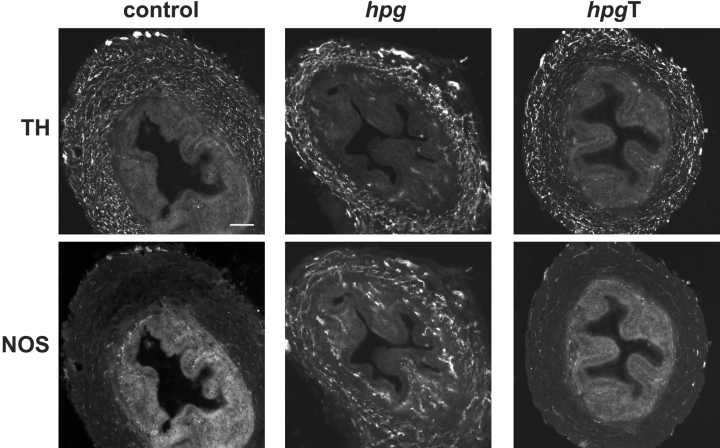
Transverse sections of vasa deferentia show noradrenergic (TH-immunoreactive) and nitrergic cholinergic (NOS-immunoreactive) nerves in the muscle and mucosal layers. TH axons are prevalent in the muscle of all three animal groups. In control and hpgT mice, NOS axons provide a sparse supply to muscle and innervate the subepithelial region. In hpg mice the NOS innervation of the muscle and the mucosa appears substantially increased. Calibration bar represents 100 μm (control, hpgT) or 50 μm (hpg).
Figure 7. Chemical characteristics of vas deferens autonomic innervation in adult control and hpg mice.
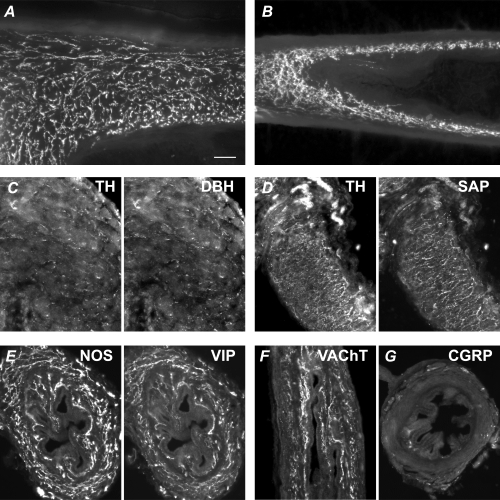
A and B, numerous axons in the muscle show catecholamine histofluorescence (glyoxylic acid method) in the control (A) and hpg (B) vas deferens. Panels C–G show vasa from hpg mice. Panels A, B, D and F show vasa sectioned longitudinally and panels C, E and G are transverse sections. C, TH-immunoreactive axons coexpress dopamine-β-hydroxylase (DBH). D, TH-immunoreactive axons coexpress the synaptic protein, synapsin (SAP). E, NOS-immunoreactive axons coexpress vasoactive intestinal peptide (VIP). F, vesicular acetylcholine transporter (VAChT)-immunoreactive axons are prevalent in the muscle and mucosa. G, sensory fibres immunolabelled for calcitonin gene-related peptide (CGRP) are rare in the muscle and mucosa, but nerve bundles can be seen in the serosa. Calibration bar represents 50 μm in all micrographs except C (20 μm).
Pelvic ganglia from hpg mice were not structurally or immunohistochemically different from controls (Fig. 8, top versus bottom panels). Quantification of TH and NOS neurons showed no difference between control, hpg and hpgT ganglia (TH: control 705 ± 24, mean ±s.e.m., hpg 723 ± 43, hpgT 608 ± 61; NOS: control 1325 ± 86, hpg 1309 ± 55, hpgT 1394 ± 31; for each substance one-way ANOVA was used to compare groups).
Figure 8. Pelvic ganglia from adult male control and hpg mice.
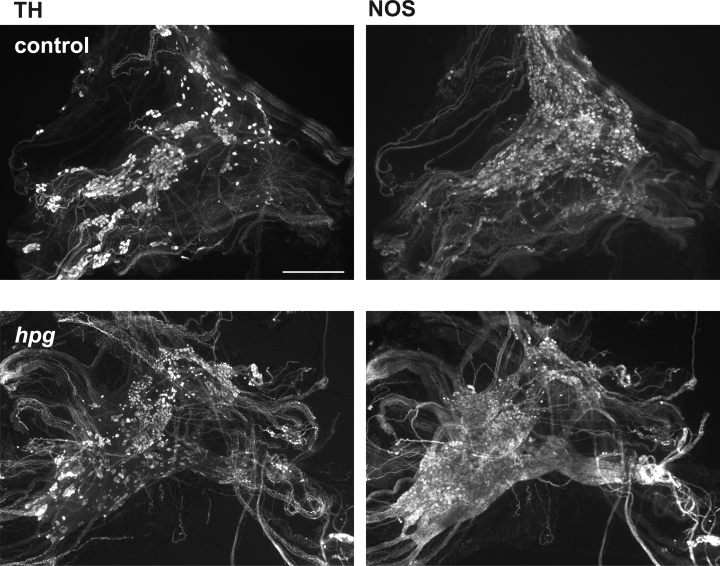
Double-staining fluorescence immunohistochemical analysis of pelvic ganglion whole mounts from adult male hpg and control mice showing separate populations of neurons immunostained for tyrosine hydroxylase (TH, noradrenergic) and nitric oxide synthase (NOS, cholinergic). Neither TH- or NOS-immunostained neurons are less prevalent in ganglia from hpg mice. Calibration bar represents 500 μm.
Prolonged androgen deprivation prevented expression of P2X1 purinoceptors, which were restored to normal levels by delayed androgen replacement
EJPs and purinergic contractions in mouse vas deferens are mediated through activation of P2X1-purinoceptors (Mulryan et al. 2000). Immunofluorescence for the P2X1 purinoceptor showed bright punctate staining of the plasma membrane for all smooth muscle cells within control vasa deferentia (Fig. 9A). No immunostaining was present in the epithelium or subepithelial tissues. All immunostaining was abolished by overnight preabsorption with the antigenic peptide (provided by the antibody supplier). Vasa deferentia from hpg mice showed almost no P2X1-immunoreactivity (Fig. 9B). Most smooth muscle cells showed very faint or no immunoreactivity but rare examples of slightly brighter fluorescence could be found (0–3 cells per section). Following testosterone replacement, vasa from hpg mice expressed levels of P2X1 comparable to controls (Fig. 9C). Quantification of immunofluorescence in vasa from control and hpg/T mice showed no significant difference between groups (mean pixel intensity: control, 115 ± 10; hpgT, 130 ± 6; n = 5 animals per group; P = 0.11)
Figure 9. P2X1 immunoreactivity in vasa deferentia.
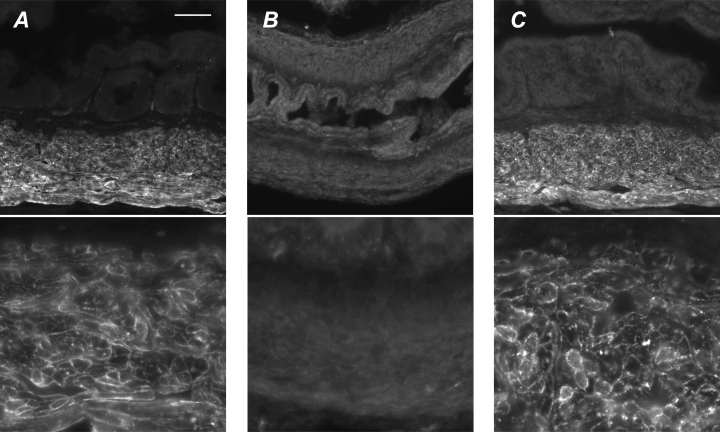
A, P2X1 immunoreactivity in smooth muscle of control vasa shows bright punctae in the plasma membrane of all smooth muscle cells but no labelling in other tissues. Occasional bright spots near the epithelium are due to autofluorescence of red blood cells. B, in hpg mice there is very pale or no P2X1 immunoreactivity associated with vas deferens smooth muscle. C, following testosterone treatment of hpg mice bright P2X1 immunoreactivity is restored to smooth muscle cells, which show bright punctate labelling in all smooth muscle cells. Calibration bar in A represents 100 μm in top row and 25 μm in bottom row.
Discussion
This study has revealed a significant temporal mismatch in the development of structural innervation and function neurotransmission in vas deferens smooth muscle following prolonged postnatal androgen deprivation. Immunohistochemical assessment of pelvic ganglion neurons and their projections to the muscle in hpg mice suggested that postnatal deprivation of androgens does not impact on the normal development of noradrenergic sympathetic innervation. This process may be androgen independent or the critical period for androgen exposure may occur before birth. In contrast, although an apparently normal sympathetic innervation is present in these mice, its ability to excite smooth muscle cells is defective. In particular, purinergic EJPs and contractions were absent and immunolabelling for P2X1 purinoceptors was almost nonexistent. Moreover, a novel nitrergic inhibitory response develops. All of these changes were reversed by delayed administration of testosterone. These results demonstrate a remarkable degree of androgen-dependent plasticity but no evidence for a critical period for development of androgen-dependent neuromuscular transmission in the vas deferens.
There was an abundant supply of noradrenergic axons within the vas deferens of hpg mice and they had a normal expression of neurotransmitter markers and synaptic proteins. This strongly suggests that much of the normal machinery for transmission is present in the presynaptic terminals, but does not discount the possibility of deficits in neurotransmitter release or defects in other aspects of synaptic function. In hpg vas deferentia there was a complete absence of evoked or spontaneous EJPs and instead IJPs were recorded. To our knowledge, IJPs have not previously been described in rodent vas deferens. As the nitric oxide synthase inhibitor l-NAME blocked the IJPs, they are most likely due to nitric oxide released from the NOS-positive parasympathetic axons that occurred at a relative high density in the media of hpg vasa deferentia. l-NAME also augmented neurally evoked contractions of hpg vasa deferentia, suggesting the nitrergic inhibitory transmission contributes functionally to the response of these tissues. This effect of l-NAME was not observed in control vasa deferentia.
In mouse vas deferens the longitudinal muscle is confined to two thin crescent shaped bands, one on either side of the tissue, and there are points between the two bands where the circular muscle is not covered by longitudinal muscle (Holman et al. 1977). In addition, the smooth muscle cells of the longitudinal muscle layer appear to be arranged into smaller bundles than those of the circular muscle layer. Because of this arrangement, it is almost certain that electrical recordings were made from both the longitudinal and circular muscle layers. Furthermore, it has been suggested, but not confirmed, that cells with variable amplitude EJPs originate from longitudinal smooth muscle cells whereas those with large amplitude EJPs originate from circular smooth muscle cells (Holman et al. 1977).
Neurally evoked contractions of hpg vasa deferentia were much smaller than those of controls. This finding can be primarily explained by smaller size of hpg vasa deferentia, as these tissues also generated much smaller contractions when the smooth muscle was directly depolarized with 60 mm K+. In addition, hpg vasa deferentia generated smaller contractions to phenylephrine but failed to respond to α,β-methylene ATP. The failure to respond to α,β-methylene ATP indicates a major difference between the smooth muscle of control and hpg vasa deferentia. This difference was confirmed by the virtual absence of immunolabelling for P2X1-purinoceptor subunits in the smooth muscle of hpg vasa deferentia.
As neurally evoked contractions of hpg vasa deferentia were almost abolished by the α1-adrenoceptor antagonist, prazosin, and augmented both by blockade of neuronal uptake of noradrenaline with DMI and by blockade presynaptic α2-adrenoceptors with rauwolscine, the sympathetic nerve terminals in these tissues must be releasing noradrenaline. In control tissues, DMI had an inhibitory effect of neurally evoked contractions that is most readily explained by an increase in α2-adrenoceptor-mediated inhibition of both noradrenaline and ATP release, leading to an overall reduction in neuroeffector transmission (see Brock et al. 1990). This explanation would also account for why, in presence of DMI, blockade of presynaptic α2-adrenoceptors produced a much larger enhancement of neurally evoked contraction in control tissues than in hpg tissues.
In hpg vasa deferentia, while prazosin almost abolished neurally evoked contractions, the muscarinic antagonist atropine also reduced neurally evoked contractions. This finding might be explained if acetylcholine released from the parasympathetic nerves acts synergistically with noradrenaline released from the sympathetic nerves to activate the smooth muscle. In support of this suggestion, it has been demonstrated that exogenously applied muscarinic agonists increase neurally evoked contractions of mouse vas deferens (Matsuno & Mita, 1992). In normal mouse vas deferens, where smooth muscle is only sparsely innervated by parasympathetic axons, blockade of muscarinic receptors only reduces neurally evoked contraction when both α1-adrenoceptors and P2X-purinoceptors are blocked (Cuprian et al. 2005).
While the smooth muscle of hpg vasa deferentia did not respond to P2X1-purinoceptor agonists, the P2-purinoceptor antagonist suramin had a facilitatory action on neurally evoked contractions of these tissues. Previous studies in mouse vas deferens have demonstrated that suramin increases both action potential evoked calcium transients in most nerve terminal varicosities (O'Connor et al. 1999) and action potential evoked release of noradrenaline (Von Kugelgen et al. 1993). These findings suggest the sympathetic nerve terminals in the mouse vas deferens possess P2-purinoceptors that are activated by endogenously released ATP (Von Kugelgen et al. 1993; O'Connor et al. 1999). Therefore the facilitatory action of suramin in hpg vasa deferentia may indicate that the sympathetic nerve terminals in these tissues release ATP that acts presynaptically to autoinhibit neurotransmitter release.
The findings of the present study suggest that postjunctional expression of P2X1-purinoceptors in the mouse vas deferens is androgen dependent. No previous studies have determined the effects of androgen deprivation on mouse vas deferens. However purinergic transmission in the mouse vas deferens only appears after 14 days of age and expression of P2X1 receptor mRNA (relative to β-actin mRNA) increases until day 46 (Liang et al. 2000). Circulating androgens also increase during this period, to reach maximum around day 55 (McKinney & Desjardin, 1973).
The effects of pre or postpubertal castration on the rat vas deferens have been fairly extensively investigated (reviewed by Keast, 2006). Neither manipulation has been reported to cause death of pelvic ganglion neurons. Prepubertal castration arrests development of the vasa deferentia whereas postpubertal castration produces a marked reduction in their size, so that they resemble those of prepubertal animals (Wakade et al. 1975; Calixto & Rae, 1981; Bustamante et al. 1989; Keast et al. 2002). Importantly, following pre- or postpubertal castration of rats, there were near parallel reductions in vas deferens weight and noradrenaline, neuropeptide Y and dopamine β-hydroxylase content of the vas deferens, suggesting that the extent of sympathetic innervation is determined primarily by the trophic influence of the tissue rather than a direct action of testosterone on neurons. However, while both pre- and postpubertal castration produced a decrease in total axonal volume within the rat vas deferens, there was an increase in the density of both sympathetic and parasympathetic axons (Keast et al. 2002). These and our current findings demonstrate the complex array of androgen actions on this tissue and its innervation during early development.
A previous study has shown that following castration of adult rats, neurally evoked contractions of their vasa deferentia were reduced in amplitude and resistant to α-adrenoceptor antagonists (MacDonald & McGrath, 1980). As the non-noradrenergic component of contraction in rat vas deferens is now known to be purinergic (Ventura, 1998), castration would appear to have little effect on the actions of neurally released ATP. This conclusion contrasts with that for the hpg vasa deferentia where purinergic transmission was absent. This difference could be due to the much earlier onset and longer duration of androgen deprivation occurring in hpg mice.
Despite the apparent absence of noradrenergic transmission in vasa deferentia from castrated adult rats, these tissues are contracted by exogenously applied α1-adrenoceptor agonists. These agents have usually produced tonic contractions (Calixto & Rae, 1981; Longhurst et al. 1989; Pupo, 1989; Longhurst, 1990; Campos et al. 2003), whereas MacDonald & McGrath (1980) reported phasic responses that resemble those in longitudinal muscle of hpg vasa deferentia. However vasa deferentia from castrated rats typically display ongoing spontaneous contractions like those observed in hpg and hpgT vasa deferentia (MacDonald & McGrath, 1980; Pupo, 1989; Campos et al. 2003). When castration was performed in adults, this spontaneous activity could be reversed by testosterone (MacDonald & McGrath, 1980). This was not the case for spontaneous activity in vas deferens of hpg mice. Therefore androgen exposure during a perinatal critical period may be essential for allowing the muscle to be quiescent.
In our study, all of the major structural and functional deficits observed in hpg mice were reversed by administration of testosterone, even though this was performed well into adulthood. Therefore despite many weeks of androgen deprivation, full function was regained and there was no postnatal critical period for development or maturation of neuroeffector transmission. Similar studies have not been reported elsewhere, although various studies have shown that replacement of testosterone following adult castration is effective in restoring tissue weight, noradrenaline and dopamine β-hydroxylase content and mechanical responses to exogenously applied contractile agents and nerve stimulation (Wakade et al. 1975; MacDonald & McGrath, 1980; Bustamante et al. 1989; Longhurst et al. 1989; Pupo, 1989; Longhurst, 1990; Campos et al. 2003). The present study has not examined the cellular mechanism of action of testosterone to ‘rescue’ normal neurotransmission in hpg mice. This could be due a direct action of testosterone on pelvic sympathetic neurons and vas deferens smooth muscle, but could also involve activation of oestrogen receptors, if testosterone is metabolized locally by aromatase to oestradiol. There is evidence for oestrogen receptor expression in male pelvic ganglia (Mäkeläet al. 2000; Salmi et al. 2001; Purves-Tyson & Keast, 2004), but aromatase expression has not been examined.
In conclusion, postnatal deprivation of androgens does not impact on neuronal survival, but is essential for the maturation of smooth muscle and neuromuscular transmission in the vas deferens. The changes produced by androgen deprivation would lead to an inability to propel sperm and seminal fluid during copulation. There may be similar defects in transmission to prostatic, epidydimal and seminal vesicle smooth muscle, where previous studies have demonstrated androgen sensitivity (Chamness et al. 1995; Hayek et al. 1999; Homma et al. 2000; Pennefather et al. 2000). Our results also indicate that postnatal androgen exposure can be delayed until adulthood, with little consequence for establishment of normal sympathetic neurotransmission.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Project Grant 300426 to J.R.K., Senior Research Fellowships to J.R.K. (358709) and J.A.B. (350904) and funding to D.J.H.), We are grateful to Prof Piers Emson for provision of the NOS antibody. We are also grateful to Melanie Yeoh, Svetlana Pianova, Nathan Marchant and Amr Al Abed for technical assistance.
References
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP. Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology. 2002;143:4922–4933. doi: 10.1210/en.2002-220493. [DOI] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC, Starke K, Wardell CF. α2adrenoceptor-mediated autoinhibition of sympathetic transmitter release in guinea-pig vas deferens studied by intracellular and focal extracellualr recording of junction potentials and currents. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:45–52. doi: 10.1007/BF00178971. [DOI] [PubMed] [Google Scholar]
- Bustamante D, Lara H, Belmar J. Changes of norepinephrine levels, tyrosine hydroxylase and dopamine-β-hydroxylase activities after castration and testosterone treatment in vas deferens of adult rats. Biol Reprod. 1989;40:541–548. doi: 10.1095/biolreprod40.3.541. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Rae GA. Influence of castration of the neonatal rat on the pharmacological reactivity of the isolated vas deferens. Biol Reprod. 1981;25:481–486. doi: 10.1095/biolreprod25.3.481. [DOI] [PubMed] [Google Scholar]
- Campos M, de Lucena Morais P, Pupo AS. Effects of castration and of testosterone replacement on α1-adrenoceptor subtypes in the rat vas deferens. Eur J Pharmacol. 2003;471:149–155. doi: 10.1016/s0014-2999(03)01822-3. [DOI] [PubMed] [Google Scholar]
- Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- Chamness SL, Ricker DD, Crone JK, Dembeck CL, Maguire MP, Burnett AL, Chang TSK. The effect of androgen on nitric oxide synthase in the male reproductive tract of the rat. Fertil Steril. 1995;63:1101–1107. [PubMed] [Google Scholar]
- Cooke B, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cuprian AM, Solanki P, Jackson MV, Cunnane TC. Cholinergic innervation of the mouse isolated vas deferens. Br J Pharmacol. 2005;146:927–934. doi: 10.1038/sj.bjp.0706357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980;3:1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- Hamill RW, Guernsey LA. Hormonal regulation of sympathetic neuron development: the effects of neonatal castration. Brain Res. 1983;313:303–307. doi: 10.1016/0165-3806(83)90228-6. [DOI] [PubMed] [Google Scholar]
- Hayek OR, Shabsigh A, Kaplan SA, Kiss AJ, Chen M-W, Burchardt T, Burchardt M, Olssen CA, Buttyan R. Castration induces acute vasoconstriction of blood vessels in the rat prostate concomitant with a reduction of prostatic nitric oxide synthase activity. J Urol. 1999;162:1527–1531. [PubMed] [Google Scholar]
- Holman ME, Taylor GS, Tomita T. Some properties of the smooth muscle of mouse vas deferens. J Physiol. 1977;266:751–764. doi: 10.1113/jphysiol.1977.sp011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma Y, Hamada K, Nakayama Y, Tsujimoto G, Kawabe K. Effects of castration on contraction and α1-adrenoceptor expression in rat prostate. Br J Pharmacol. 2000;131:1454–1460. doi: 10.1038/sj.bjp.0703706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- Keast JR, Gleeson RJ, Shulkes A, Morris MJ. Maturational and maintenance effects of testosterone on terminal axon density and neuropeptide expression in the rat vas deferens. Neuroscience. 2002;112:391–398. doi: 10.1016/s0306-4522(02)00077-5. [DOI] [PubMed] [Google Scholar]
- Liang SX, D'Arbe M, Phillips WD, Lavidis NA. Development of fast purinergic transmission in the mouse vas deferens. Synapse. 2000;37:283–291. doi: 10.1002/1098-2396(20000915)37:4<283::AID-SYN5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Longhurst PA. The effects of testosterone or insulin treatment on contractile responses of the rat vas deferens following castration or streptozotocin-induced mellitus. Gen Pharmacol. 1990;21:427–434. doi: 10.1016/0306-3623(90)90693-g. [DOI] [PubMed] [Google Scholar]
- Longhurst PA, Brotcke TP, Burrell CL, Belis JA. Comparison of the effects of castration and streptozotocin-induced diabetes mellitus on contractile responses of the rat vas deferens. Pharmacology. 1989;38:253–262. doi: 10.1159/000138544. [DOI] [PubMed] [Google Scholar]
- MacDonald A, McGrath JC. The effects of castration on neurotransmission in the rat vas deferens. Br J Pharmacol. 1980;69:49–58. doi: 10.1111/j.1476-5381.1980.tb10882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Estrogen effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McKinney TD, Desjardin C. Postnatal development of the testis, fighting behavior, and fertility in house mice. Biol Reprod. 1973;9:279–294. doi: 10.1093/biolreprod/9.3.279. [DOI] [PubMed] [Google Scholar]
- Mäkelä S, Strauss L, Kuiper G, Valve E, Salmi S, Santti R, Gustafsson J-A. Differential expression of estrogen receptors α and β in adult rat accessory sex glands and lower urinary tract. Mol Cell Endocrinol. 2000;170:219–229. doi: 10.1016/s0303-7207(00)00441-x. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Mita S. Involvement of the muscarinic receptors in the postsynaptic potentiation of neurogenic twitch contraction in the mouse vas deferens. Life Sci. 1992;50:799–806. doi: 10.1016/0024-3205(92)90185-r. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Hamill RW. Gonadal hormone regulation of neurotransmitter synthesizing enzymes in the developing hypogastric ganglion. Brain Res. 1986;383:38–46. doi: 10.1016/0006-8993(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- O'Connor SC, Brain KL, Bennett MR. Individual sympathetic varicosities possess different sensitivities to α2 and P2 receptor agonists in mouse vas deferens. Br J Pharmacol. 1999;128:1739–1753. doi: 10.1038/sj.bjp.0702984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather JN, Lau WA, Mitchelson F, Ventura S. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol. 2000;20:193–206. doi: 10.1046/j.1365-2680.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- Pupo AS. Functional effects of castration on α1-adrenoceptors in rat vas deferens. Eur J Pharmacol. 1989;351:217–233. doi: 10.1016/s0014-2999(98)00315-x. [DOI] [PubMed] [Google Scholar]
- Purves-Tyson TD, Keast JR. Rapid actions of estradiol on cyclic AMP response-element binding protein phosphorylation in dorsal root ganglion neurons. Neuroscience. 2004;129:629–627. doi: 10.1016/j.neuroscience.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Salmi S, Santti R, Gustafsson JA, Makela S. Co-localization of androgen receptor with estrogen receptor β in the lower urinary tract of the male rat. J Urol. 2001;166:674–677. [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Singh J, Handelsman DJ. Imprinting by neonatal sex steroids on the structure and function of the mature mouse prostate. Biol Reprod. 1999;61:200–208. doi: 10.1095/biolreprod61.1.200. [DOI] [PubMed] [Google Scholar]
- Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136:5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- Ventura S. Autoinhibition, sympathetic cotransmission and biphasic contractile responses to trains of nerve stimulation in the rodent vas deferens. Clin Exp Pharmacol Physiol. 1998;25:965–973. doi: 10.1111/j.1440-1681.1998.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Von Kugelgen I, Kurz K, Starke K. Axon terminal P2-purinoceptors in feedback control of sympathetic transmitter release. Neuroscience. 1993;56:263–267. doi: 10.1016/0306-4522(93)90330-i. [DOI] [PubMed] [Google Scholar]
- Wakade AR, Garcia AG, Kirkepar SM. Effect of castration on the smooth muscle cells of the internal sex organs of the rat: influence of the smooth muscle on the sympathetic neurons innervating the vas deferens, seminal vesicle and coagulating gland. J Pharmacol Exp Ther. 1975;193:424–434. [PubMed] [Google Scholar]
- Wanigasekara Y, Airaksinen MS, Heuckeroth RO, Milbrandt J, Keast JR. Neurturin signalling via GFRα2 is essential for innervation of glandular but not muscle targets of sacral parasympathetic ganglion neurons. Mol Cell Neurosci. 2004;25:288–300. doi: 10.1016/j.mcn.2003.10.019. [DOI] [PubMed] [Google Scholar]


