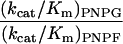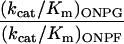Table 1.
Kinetic constants for the native and evolved enzymes
| Substrate | Kinetic constant | Native galactosidase | Evolved fucosidase | |
|---|---|---|---|---|
| PNPG | kcat, s−1 | 268 | 30.9 | |
| Km, mM | 0.04 | 0.18 | ||
| kcat/Km, mM−1⋅s−1 | 6,700 | 172 | ||
| PNPF | kcat, s−1 | 209 | 96.6 | |
| Km, mM | 31 | 1.5 | ||
| kcat/Km, mM−1⋅s−1 | 6.7 | 64.4 | ||
| Specificity |
|
1,000 | 2.7 | |
| ONPG | kcat, s−1 | 765 | 14.5 | |
| Km, mM | 0.11 | 0.11 | ||
| kcat/Km, mM−1⋅s−1 | 6,950 | 132 | ||
| ONPF | kcat, s−1 | — | 24.1 | |
| Km, mM | — | 0.55 | ||
| kcat/Km, mM−1⋅s−1 | (2)* | 43.9 | ||
| Specificity |
|
3,200 | 3.0 |
The native galactosidase and the evolved fucosidase were purified, and the enzymes were assayed on four different substrates.
The kcat/Km value for the native galactosidase on ONPF was estimated to be about 2 mM−1⋅s−1 by measuring the hydrolysis rate relative to that of ONPG.