Figure 4. Phosphorylation of hERG is increased by activation of PKC.
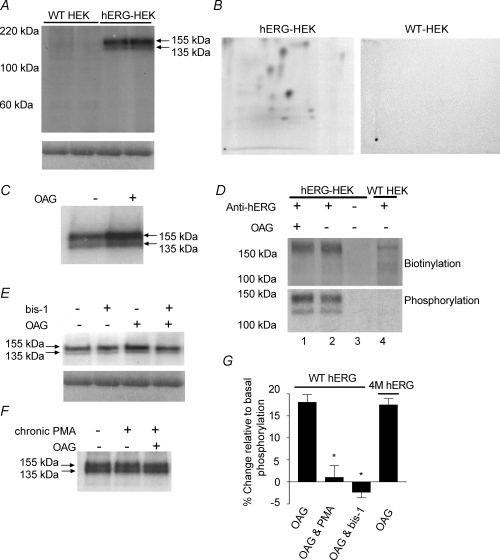
Phospho-proteins were immunoprecipitated from WT-HEK cells and hERG-HEK cells using anti-hERG antibody. A, upper panel, representative autoradiograph showing phospho-proteins were only pulled down from hERG-expressing cells. Samples were run in duplicate to demonstrate reproducibility of results. The molecular masses of the two bands correspond with the immature (135 kDa) and mature (155 kDa) forms of hERG. Both hERG proteins are clearly phosphorylated in untreated cells. The lower panel shows Coomassie blue staining of the gel before drying and demonstrates there is equal antibody loading and immunoprecipitation across lanes. B, representative phospho-peptide maps following tryptic digest and separation by 2D electrophoresis of phospho-proteins immunoprecipitated from untreated hERG-HEK cells and WT-HEK cells. The hERG-HEK cell plate was exposed for 10 days and the WT HEK cell plate for longer (14 days). Phospho-peptides can only be detected from hERG expressing cells. C, representative autoradiograph showing the increase of phosphorylation in cells treated with 10 μm OAG for 5 min. D, quantification of biotinylation and phosphorylation of immunoprecipitated proteins. Protein samples transferred to nitrocellulose were first probed with streptavidin (upper panel), and subsequently exposed to autoradiography film for phospho-protein detection (lower panel). The streptavidin labelling was equivalent in OAG treated cells (lane 1) and untreated cells (lane 2), indicating equal amounts of immunoprecipitated hERG in each lane. Comparison of hERG phosphorylation levels in lanes 1 and 2 indicate there was a 17% increase over basal levels with OAG stimulation. No phospho-proteins or streptavidin labelled proteins were detected if anti-hERG serum was not added to the lysates (lane 3). Faint streptavidin-labelled bands were detected in some WT-HEK lysates (lane 4), but the proteins were not phosphorylated and were present in only small quantities. Similar results were seen in two other experiments. E, bis-1 inhibits OAG-dependent increase of hERG subunit phosphorylation. Bis-1 at 3 μm did not alter basal phosphorylation, but blocked the increase of phosphorylation by OAG. Lower panels, Coomassie blue staining of gel indicating equal immunoprecipitation across lanes. F, incubation of cells with 1 μm PMA for 24 h to down-regulate PKC isotypes also prevented the increase in phosphorylation by OAG. There was also no change in phosphorylation in untreated versus PMA-treated cells. G, mean change of phosphorylation for WT hERG and 4M-hERG (a functional channel lacking PKA phosphorylation). The percentage change of phosphorylation relative to basal levels was measured in untreated cells. Chronic PMA preincubation and bis-1 treatment abolished the increase of WT hERG phosphorylation in response to OAG. In cells transiently expressing 4M-hERG, the phosphorylation response to OAG was the same as WT hERG. Measurements for each experimental treatment have been repeated a minimum of 3 times. *Significantly different from WT hERG response to OAG alone (P < 0.05).
