Abstract
We sought to determine if resetting of the carotid-vasomotor baroreflex function curve during exercise is modulated by changes in central blood volume (CBV). CBV was increased during exercise by altering: (1) subject posture (supine versus upright) and (2) pedal frequency (80 versus 60 revolutions min−1 (r.p.m.)); while oxygen uptake (V˙O2) was kept constant. Eight male subjects performed three exercise trials: upright cycling at 60 r.p.m. (control); supine cycling at 60 r.p.m. (SupEX) and upright cycling at 80 r.p.m. to enhance the muscle pump (80EX). During each condition, carotid baroreflex (CBR) function was determined using the rapid neck pressure (NP) and neck suction (NS) protocol. Although mean arterial pressure (MAP) was significantly elevated from rest (88 ± 2 mmHg) during all exercise conditions (P < 0.001), the increase in MAP was lower during SupEX (94 ± 2 mmHg) and 80EX (95 ± 2 mmHg) compared with control (105 ± 2 mmHg, P < 0.05). Importantly, the blood pressure responses to NP and NS were maintained around these changed operating points of MAP. However, in comparison to control, the carotid-vasomotor baroreflex function curve was relocated downward and leftward when CBV was increased during SupEX and 80EX. These alterations in CBR resetting occurred without any differences in V˙O2 or heart rate between the exercise conditions. Thus, increasing CBV and loading the cardiopulmonary baroreflex reduces the magnitude of exercise-induced increases in MAP and CBR resetting. These findings suggest that changes in cardiopulmonary baroreceptor load influence carotid baroreflex resetting during dynamic exercise.
Over the past 25 years both animal and human experimentation have verified that the arterial baroreflex is reset from rest to exercise in direct relation to work intensity (Raven et al. 2006). Many of the studies that have confirmed the resetting of the arterial baroreflex have indicated that activation of central command or the exercise pressor reflex independently, or in combination, are required for the arterial baroreflex to be reset during exercise (Iellamo et al. 1997; Norton et al. 1999; Gallagher et al. 2001b,c; McIlveen et al. 2001; Querry et al. 2001; Ogoh et al. 2002; Smith et al. 2003; Gallagher et al. 2006). It has been argued that greater activation of central command and the exercise pressor reflex elicited by increasing the exercise intensity or exercising muscle mass leads to a greater pressor response (Fadel et al. 2003; Gallagher et al. 2006; Raven et al. 2006). Although baroreflex resetting indeed occurs in an intensity-dependent manner, increases in work intensity or the addition of muscle mass and their subsequent increases in central command and the exercise pressor reflex may not be the only determinants of the blood pressure response and the degree of baroreflex resetting during exercise.
In a recent study, when leg-cycling exercise was added to arm-cranking exercise, arterial blood pressure was reduced below that of arm exercise alone (Volianitis & Secher, 2002). Moreover, the addition of leg exercise to arm exercise resulted in the operating point of the carotid-vasomotor reflex relocating to a lower blood pressure than during arm exercise alone (Volianitis et al. 2004). Thus, despite engaging a larger muscle mass and performing more work with arm plus leg exercise, arterial blood pressure and the operating point of the carotid-vasomotor reflex were reduced. Collectively, these findings suggest that changes in central blood volume (CBV), produced by the muscle-pumping enhancement of venous return resulting from the leg exercise, greatly influences the exercise-induced blood pressure response and the locus of the operating point of the carotid-vasomotor reflex. Thus, inputs from the receptor populations sensing CBV, which are generally accepted to be defined as cardiopulmonary baroreceptors, may influence arterial baroreflex control during exercise. Unfortunately, these previous investigations were confounded by the fact that different energy expenditures were utilized for the arm, leg, and combined arm–leg exercise conditions, leading to significant differences in exercising heart rates (Volianitis & Secher, 2002; Volianitis et al. 2004), thereby suggesting that the degree of central command and exercise pressor reflex activation were different among exercise conditions. Additional experimental protocols designed to examine the influence of CBV on the carotid baroreflex have utilized lower-body-negative pressure and the infusion of serum albumin to unload and load the cardiopulmonary baroreceptors, respectively (Ogoh et al. 2006a,b). Although these studies have demonstrated that changes in CBV altered arterial baroreflex sensitivity, these experimental paradigms were also confounded by significant differences in exercising heart rates compared to control exercise, perhaps because of somewhat supra-physiological alterations in CBV.
Taking the limitations of previous studies into consideration, the present study was specifically designed to increase CBV during dynamic exercise while keeping oxygen consumption constant in an attempt to maintain central command and exercise pressor reflex activation similar. We reasoned that this approach would maintain similar energy expenditures and exercising heart rates, while solely manipulating CBV and cardiopulmonary baroreceptor load. This was accomplished by changing posture from the upright to supine position and by increasing pedal frequency to enhance the muscle pump during cycling exercise. Carotid baroreflex function curves were constructed using the variable-pressure neck collar technique during upright cycling at 60 r.p.m. (control) and the derived baroreflex function parameters were compared to those obtained during supine cycling at 60 r.p.m. (SupEX) and upright cycling at 80 r.p.m. (80EX) where CBV was enhanced. We hypothesized that increases in CBV, and thus cardiopulmonary baroreceptor load, would modulate baroreflex resetting during dynamic exercise such that in comparison to control exercise the carotid-vasomotor baroreflex function curve would be reset downward and leftward when CBV was elevated.
Methods
Eight men with a mean age 29 ± 1 years, height 175 ± 2 cm, and weight 76 ± 3 kg (mean ± s.e.m.) were recruited for voluntary participation in the present study. Each subject received a verbal and written explanation of the study objectives, measurement techniques, and the risks and benefits associated with the investigation, and provided written informed consent as approved by the Institutional Review Board at the University of Missouri and the Research and Development committee at the Harry S. Truman Memorial Veterans' Hospital. All experiments were performed in accordance with the Declaration of Helsinki. All subjects were free of any known cardiovascular or respiratory diseases and were currently not taking medications. Prior to the experiments each subject was familiarized with the equipment and the experimental protocol. The subjects were requested to abstain from caffeinated beverages for 12 h and strenuous physical activity and alcohol intake for at least 24 h prior to any testing.
Measurements
All studies were performed at a constant room temperature between 23 and 24°C with external stimuli minimized. Heart rate (HR) was monitored using a lead II electrocardiogram (ECG). Beat-to-beat arterial blood pressure (ABP) was monitored non-invasively by a servo-controlled finger photoplethysmograph (Finometer, Finapres Medical Systems, Amsterdam, the Netherlands) placed on the middle finger of the left hand. Previous work has demonstrated that the changes in mean arterial pressure (MAP) measured by photoplethysmography are not different from direct arterial blood pressure measurements both at rest and during dynamic exercise (Idema et al. 1989; Shi et al. 1993; Imholz, 1996). However, because photoplethysmography may not be as accurate for absolute blood pressure measurements, an automated sphygmomanometer (SunTech, Medical Instruments Raleigh, NC, USA) was used to validate the Finometer measurements. Thus, blood pressure was measured every 45 s by auscultation of the brachial artery of the right arm before and during dynamic exercise. MAP was calculated as diastolic blood pressure plus one-third pulse pressure. The beat-to-beat change in MAP measured by photoplethysmography was uniformly corrected to the absolute blood pressure recorded via sphygmomanometry and used for estimating baroreflex function. Impedance cardiography (Minnesota Impedance Cardiograph, Model 304B, Surcom Inc., Minneapolis, MN, USA) was used to measure thoracic electrical impedance (TI). Thoracic admittance (1/TI) was used as index of central blood volume (CBV), as previously described (Cai et al. 2000). All signals were connected to Powerlab (ADInstruments, Bella Vista, NSW, Australia) interfaced with a personal computer equipped with customized data acquisition software for the beat-to-beat recording of physiological variables. The ECG signal, the arterial pressure waveforms, and TI were sampled at 1 kHz, and real-time beat-to-beat values of HR, mean (MAP), systolic (SBP) and diastolic blood pressures (DBP) were stored for offline analysis. The standard 6–20 Borg scale was used to obtain individual ratings of perceived exertion (RPE) at the end of each exercise trial (Borg, 1982).
Respiratory measurements to assess the rate of oxygen uptake (V˙O2) were collected using breath-by-breath open circuit spirometry (TrueMax 2400, ParvoMedics, Salt Lake City, UT). Briefly, subjects respired through a mouthpiece attached to a volume transducer while gases were continuously sampled for analysis of fractional concentrations of O2, carbon dioxide and nitrogen. Prior to each experiment, the system was calibrated using known standard gases.
Experimental protocols
On the experimental day, the subjects arrived at the laboratory at least 2 h after a light meal. Subjects were seated in a semirecumbent position on a medical exam table equipped with a cycle ergometer (Angio V2, Lode, Groningen, Netherlands). The subjects were fitted with a malleable lead neck collar that encircled the anterior two thirds of the neck for the application of neck pressure (NP) and neck suction (NS). Following instrumentation, subjects rested quietly while baseline measurements were acquired for 10 min, after which carotid baroreflex (CBR) function was determined using the rapid NP and NS protocol (Pawelczyk & Raven, 1989) during a 10–15 s breath-hold at end-expiration. Twelve consecutive pulses (range: +40 to −80 mmHg) of 500 ms duration were delivered to the carotid sinus precisely 50 ms after the R wave of the ECG, to elicit maximum baroreflex responses (Eckberg, 1977). Four to five trains of the rapid NP and NS protocol were performed and separated by a minimum of 45 s. After the rapid NP and NS protocol at rest, subjects performed the control exercise bout.
The control cycling bout began with a low workload (20–30 W), which was then adjusted to elicit a target HR of 100 beats min−1, while pedal frequency was maintained at 60 r.p.m. A 3–5-min period was used to adjust the workload to elicit the target HR. After the target HR was achieved, subjects exercised for 6 min to assure steady-state which was assessed by the continuous HR and V˙O2 measures. Once steady-state conditions were confirmed, V˙O2 measurements were ended and four to five trains of the rapid NP and NS protocol were applied without a breath-hold (Eckberg et al. 1980) with a minimum of 30 s between each rapid NP and NS trial. The steady-state V˙O2 from the control exercise was used as the target V˙O2 for the subsequent exercise trials. As such, during the 80EX and SupEX conditions we used V˙O2 as our target workload measure instead of HR.
Following a 30–40 min rest period, to enable sufficient recovery, subjects repeated the exercise protocol at a workload that elicited the same V˙O2 as upright cycling at 60 r.p.m. (control), but during this bout subjects were asked to maintain a pedal frequency of 80 r.p.m. (80EX). The increase in pedal frequency was used to enhance the effect of the muscle pump and increase CBV (Gotshall et al. 1996). The exercise bout began with a low workload (20–30 W), which was then adjusted to elicit the target V˙O2 of the control bout, while pedal frequency was maintained at 80 r.p.m. A 3–5 min period was used to adjust the workload to elicit the target V˙O2. Once the target V˙O2 was achieved, subjects exercised for 6 min to assure steady-state, which was assessed by the continuous V˙O2 and HR measures. At this point, V˙O2 measurements were ended and CBR function was assessed. After this exercise bout subjects rested in the semirecumbent position for 15 min and were then moved to the supine position for an additional 15 min to assure adequate recovery. The movement of the subject to the supine position was used to increase CBV and load the cardiopulmonary baroreceptors. CBR function testing was repeated at rest and during supine cycling at 60 r.p.m. at a workload that elicited the same V˙O2 as control exercise (upright cycling at 60 r.p.m.). Due to the experimental setup and logistics, the supine exercise was typically performed last. However, in order to determine if there was any order effect, we performed two studies in which SupEX was performed before 80EX. Similar results were obtained. By design, upright cycling at 60 r.p.m. (control) was always performed first. This was necessary to establish the V˙O2 that elicited a HR of 100 beats min−1, which was then used to determine the workloads performed during SupEX and 80EX.
CBR function curves
The carotid-cardiac (HR) and the carotid-vasomotor (MAP) responses were evaluated by plotting the changes in HR and MAP, respectively, against the estimated carotid sinus pressure (ECSP), which was calculated as MAP minus neck chamber pressure. The CBR stimulus–response data were fitted to the logistic model described by Kent et al. (1972). This function incorporates the following equation:
where HR or MAP is the dependent variable, ECSP is the estimated carotid sinus pressure, A1 is the range of response of the dependent variable (maximum – minimum), A2 is the gain coefficient (i.e. slope), A3 is the carotid sinus pressure required to elicit an equal pressor and depressor response (centring point), and A4 is the minimum response of HR or MAP. The data were fitted to this model by non-linear least-squares regression (using a Marquardt–Levenberg algorithm), which minimized the sum of squares error term to predict a curve of ‘best fit’ for each set of raw data. The coefficient of variation for the overall fit of this model to the individual responses was 18% (Potts et al. 1993). The gain was calculated from the first derivative of the logistic function and the maximal gain (Gmax) was applied as the index of carotid baroreflex responsiveness. Threshold (THR), the point where no further increase in the dependent variable occurred despite reductions in ECSP, and saturation (SAT), the point where no further decrease in the dependent variable occurred despite increases in ECSP, were calculated as the maximum and minimum second derivatives, respectively, of the logistic function curve. For calculation of THR and SAT, we applied equations described by McDowall & Dampney (2006):
and
These calculations of THR and SAT are the carotid sinus pressure at which HR or MAP is within 5% of the upper or lower plateau of the sigmoid function.
Thoracic admittance
Changes in thoracic admittance (Δ1/TI) were used as an index of changes in CBV (Cai et al. 2000). The changes in thoracic admittance were calculated by comparing 1 min segments taken immediately preceding exercise and during steady-state exercise conditions just prior to the assessment of CBR function. In order to account for the full effect of altering posture on CBV, the change in thoracic admittance during SupEX was compared to upright rest.
Statistical analysis
Statistical comparisons of physiological variables and CBR function curve parameters were made utilizing one-way repeated-measures analyses of variance (ANOVA). After ANOVA analyses, a Student–Newman–Keuls test was employed post hoc to identify significant differences between each condition. Statistical significance was set at P < 0.05, and results are presented as means ± s.e.m. Analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS Inc., Chicago, IL, USA).
Results
Physiological responses to postural changes at rest
At rest, a change in position from upright to supine increased thoracic admittance ((+11.8 ± 3.0) ×10−4 S P = 0.006) and decreased MAP (−6 ± 1 mmHg, P = 0.002) and DBP (−7 ± 2 mmHg, P = 0.002) (Table 1). However, there were no significant differences in SBP (−4 ± 1, P = 0.274) or HR (−2 ± 4 beats min−1, P = 0.588) between the two positions.
Table 1.
Cardiovascular variables at rest and during cycling exercise
| UP REST | Control | 80EX | SU REST | SupEX | |
|---|---|---|---|---|---|
| HR (beats min−1) | 59 ± 4 | 99 ± 1* | 95 ± 3* | 57 ± 5†‡ | 93 ± 1*§ |
| SBP (mmHg) | 120 ± 3 | 156 ± 6* | 146 ± 6*† | 116 ± 6†‡ | 147 ± 6*†§ |
| DBP (mmHg) | 72 ± 3 | 79 ± 3* | 70 ± 3† | 65 ± 2*†‡ | 67 ± 2*† |
| MAP (mmHg) | 88 ± 2 | 105 ± 2* | 95 ± 3*† | 82 ± 2*†‡ | 94 ± 2*†§ |
| PP (mmHg) | 48 ± 4 | 78 ± 8* | 75 ± 7* | 52 ± 4†‡ | 80 ± 7*§ |
| V˙O2 (l min−1) | 0.235 ± 0.013 | 0.961 ± 0.092* | 0.957 ± 0.080* | 0.241 ± 0.013†‡ | 0.942 ± 0.096*§ |
| V˙O2 (ml kg−1 min−1) | 3.08 ± 0.10 | 12.62 ± 1.01* | 12.59 ± 0.88* | 3.16 ± 0.12†‡ | 12.37 ± 1.09*§ |
| RPE | 11.3 ± 0.4 | 13.0 ± 0.5† | 12.3 ± 0.7 | ||
| Workload (W) | 58 ± 9 | 44 ± 8† | 55 ± 8†‡ |
Values are means ± s.e.m. UP, upright; SU, supine; Control, upright cycling exercise at 60 r.p.m.; 80EX, upright cycling exercise at 80 r.p.m.; SupEX, supine cycling exercise at 60 r.p.m.; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; V˙O2, oxygen uptake; RPE, rating of perceived exertion.
Different from UP REST, P < 0.05
different from control, P < 0.05
different from 80EX, P < 0.05
different from SU REST, P < 0.05.
Physiological responses during exercise
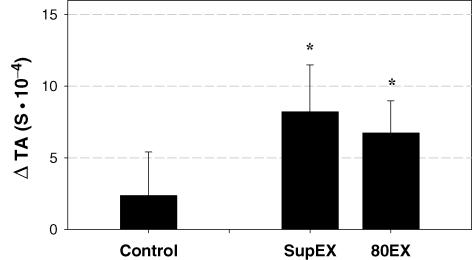
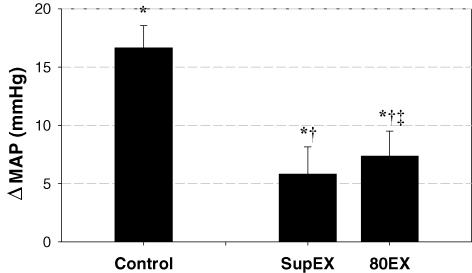
As determined a priori, each subject performed three bouts of cycling exercise at the same V˙O2 (Table 1). To accomplish this goal, the exercise workloads were slightly but significantly lower during SupEX and 80EX (55 ± 8 W and 44 ± 8 W, respectively) compared to control (58 ± 9 W), i.e. upright exercise at 60 r.p.m. (Table 1). Importantly, exercising heart rates were not different between the three exercise conditions. However, RPE was significantly greater during 80EX compared to control (P = 0.005). Although thoracic admittance increased from upright rest during control exercise, this increase was not significant ((2.37 ± 3.04) ×10−4 S, P = 0.460). In contrast, as expected, thoracic admittance was significantly elevated from upright rest during both SupEX ((8.21 ± 3.27) ×10−4 S, P = 0.040) and 80EX ((6.74 ± 2.25) ×10−4 S, P = 0.020) (Fig. 1). During all exercise trials, SBP, DBP and MAP were significantly increased (P < 0.001). However, compared with control, the increases in blood pressure were reduced during SupEX and 80EX (Table 1 and Fig. 2). Pulse pressures were not different between the three exercise conditions (P = 0.281, Table 1).
Figure 1. Changes in thoracic admittance (TA) from the upright rest period preceding each condition.
This analysis accounted for slight changes in the TA signal caused by positional changes. Values are means ± s.e.m. Control, upright cycling at 60 r.p.m.; SupEX, supine cycling at 60 r.p.m.; 80EX, upright cycling at 80 r.p.m. TA is 1/thoracic impedance; S, siemens. *Different from upright rest, P < 0.05.
Figure 2. Summary data showing changes in mean arterial pressure (MAP) from upright rest during each exercise condition.
The change in MAP in both the supine (SupEX) and higher pedalling frequency (80EX) exercise conditions was significantly lower than control upright exercise at 60 r.p.m. Values are means ± s.e.m. *Different from upright rest, P < 0.05; †different from control exercise, P < 0.05; ‡different from SupEX, P < 0.05.
Resetting of the carotid-vasomotor stimulus–response curve during exercise
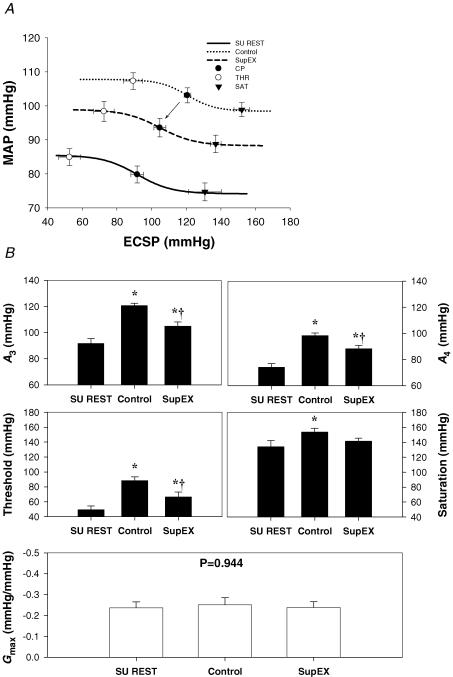
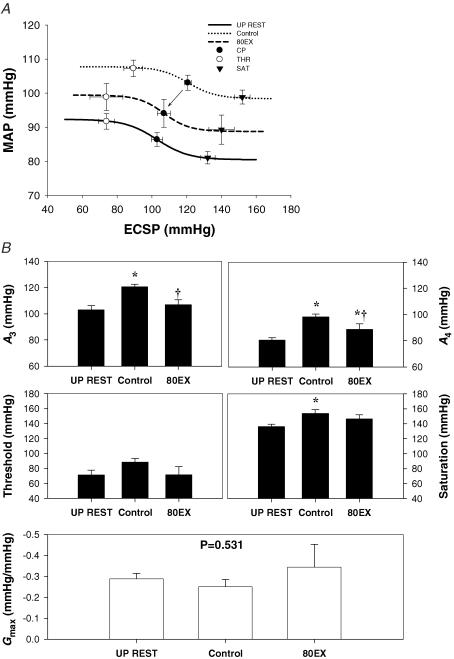
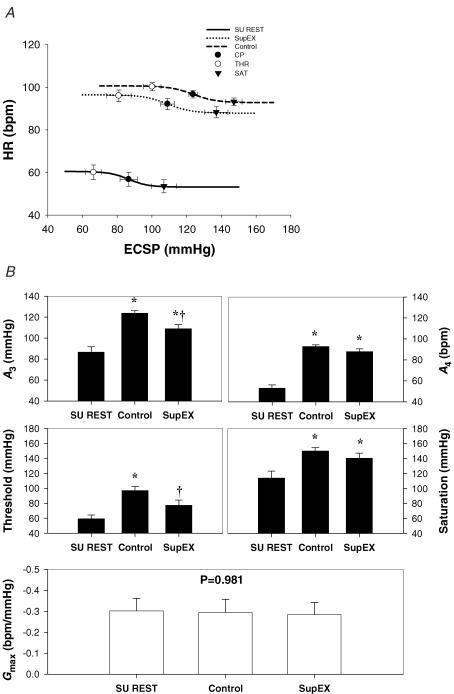
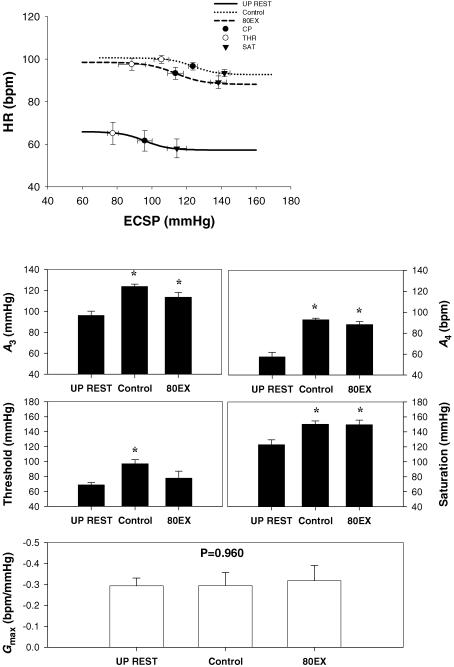
The logistic model parameters describing the carotid-vasomotor baroreflex function curves at rest and during the three exercise conditions are presented in Figs 3 and 4. The response range and maximal gain were unaltered during all exercise bouts. During control exercise, the operating point, centring point (A3), and minimum response (A4), and saturation were significantly elevated compared to upright rest, indicative of an upward and rightward resetting of the carotid-vasomotor baroreflex function curve (Figs 3 and 4). In comparison, SupEX (Fig. 3) resulted in a significant attenuation in the exercise-induced increase in the operating point, centring point, minimum response, and threshold, resulting in the carotid-vasomotor baroreflex function curve being relocated downward and leftward in relation to control exercise. Similar results were found for 80EX (Fig. 4).
Figure 3. Reflex responses in mean arterial pressure (MAP) (carotid-vasomotor) to NP/NS stimulation of the carotid sinus baroreceptors during supine rest (SU REST), supine cycling (SupEX) and upright cycling at 60 r.p.m. (Control).
A, the baroreflex function curves where the lines, symbols and bars denote actual group data for all subjects. • represents the centring point or point of maximal gain; ○ represents the carotid sinus pressure threshold, and ▾ represents the carotid sinus pressure saturation. B, summary data for the logistic model parameters of A3 (centring point), A4 (the minimal response of MAP), threshold, saturation, and maximal gain (Gmax) of the baroreflex function curves. Values are mean ± s.e.m. *Different from rest, P < 0.05; †different from control exercise, P < 0.05. Similar to the upright position during SupEX the carotid-vasomotor curve was reset upward and rightward. However, this curve was located downward and leftward compared with control.
Figure 4. Reflex responses in mean arterial pressure (MAP) (carotid-vasomotor) to NP/NS stimulation of the carotid sinus baroreceptors during upright rest (UP REST), upright cycling exercise at 80 r.p.m. (80EX) and 60 r.p.m. (Control).
A, the baroreflex function curves where the lines, symbols and bars denote actual group data for all subjects as described in Fig. 3. B, summary data for the logistic model parameters of A3 (centring point), A4 (the minimal response of MAP), threshold, saturation, and maximal gain (Gmax) of the baroreflex function curves. Values are mean ± s.e.m. *Different from rest, P < 0.05; †different from control exercise, P < 0.05. When central blood volume was increased by increasing pedal frequency, the carotid-vasomotor baroreflex function curve was located downward and leftward compared to control exercise.
Resetting of the carotid-cardiac stimulus–response curve during exercise
The logistic model parameters describing the carotid-cardiac baroreflex function curves at rest and during the three exercise conditions are presented in Figs 5 and 6. In contrast to changes in the carotid-vasomotor logistic parameters and baroreflex function curves, the carotid-cardiac baroreflex function curve remained largely unaffected by changes in position (SupEX) and pedal frequency (80EX). However, there was a tendency for the carotid-cardiac baroreflex function curve to be shifted to the left during SupEX and 80EX, largely due to changes in estimated carotid sinus pressure at the operating point and threshold (Figs 5 and 6). Overall, the carotid-cardiac baroreflex function curve was relocated upward and rightward from rest without changes in the maximal gain under all exercise conditions.
Figure 5. Reflex responses in heart rate (HR) (carotid-cardiac) to NP/NS stimulation of the carotid sinus baroreceptors during supine rest (SU REST), supine cycling (SupEX) and upright cycling at 60 r.p.m. (Control).
A, the baroreflex function curves where the lines, symbols and bars denote actual group data for all subjects as described in Fig. 3. B, summary data for the logistic model parameters of A3 (centring point), A4 (the minimal response of MAP), threshold, saturation, and maximal gain (Gmax) of the baroreflex function curves. Values are mean ± s.e.m. *Different from rest, P < 0.05; †different from control exercise, P < 0.05. The carotid-cardiac baroreflex function curve remained largely unaffected by changes in position (SupEX). However, there was a tendency for the curve to be shifted to the left during SupEX, largely due to changes in estimated carotid sinus pressure at the operating point, centring point and threshold.
Figure 6. Reflex responses in heart rate (HR) (carotid-cardiac) to NP/NS stimulation of the carotid sinus baroreceptors during upright rest (UP REST), upright cycling exercise at 80 r.p.m. (80EX) and 60 r.p.m. (Control).
A, the baroreflex function curves where the lines, symbols and bars denote actual group data for all subjects as described in Fig. 3. B, summary data for the logistic model parameters of A3 (centring point), A4 (the minimal response of MAP), threshold, saturation, and maximal gain (Gmax) of the baroreflex function curves. Values are mean ± s.e.m. *Different from rest, P < 0.05. The carotid-cardiac baroreflex function curve remained largely unaffected by changes in pedal frequency.
Discussion
The present investigation provides novel information regarding the importance of changes in CBV and cardiopulmonary baroreceptor load on carotid baroreflex resetting during dynamic exercise. In comparison to control exercise (upright at 60 r.p.m.), the carotid-vasomotor baroreflex function curve was relocated downward and leftward when CBV was increased by having the subjects perform the cycling exercise in the supine position, and also by having the subjects increase their pedal frequency from 60 r.p.m. to 80 r.p.m. Importantly, both manoeuvres resulted in significant reductions in the exercising blood pressure despite similar increases in oxygen uptake and heart rate. Thus, increasing CBV and loading the cardiopulmonary baroreceptors reduces the magnitude of exercise-induced increases in MAP and CBR resetting. Collectively, these findings suggest that changes in cardiopulmonary baroreceptor input influence CBR resetting during dynamic exercise.
Baroreflex resetting
Numerous investigations in humans have demonstrated that the arterial baroreflex is reset and continues to operate around the prevailing blood pressure generated during physical activity (Raven et al. 2006). This resetting occurs in direct relation to the intensity of the exercise from rest to maximal exercise (Potts et al. 1993; Papelier et al. 1994; Norton et al. 1999; Ogoh et al. 2003b). Rowell & O'Leary (1990) formulated a working hypothesis of arterial baroreflex resetting during exercise and its regulation of sympathetic nerve activity. They proposed that two neural mechanisms, central command and the exercise pressor reflex, which are activated during exercise, mediate the resetting of the arterial baroreflex. Recently, in order to directly test this hypothesis, several studies (Iellamo et al. 1997; Gallagher et al. 2001b,c; McIlveen et al. 2001; Querry et al. 2001; Ogoh et al. 2002; Smith et al. 2003; Gallagher et al. 2006) have attempted to manipulate central command or exercise pressor reflex input during exercise. The findings from these studies indicate that central command relocates both the carotid-cardiac and carotid-vasomotor baroreflex function curves upward on the response arm and rightward to a higher arterial pressure (Raven et al. 2006). Similarly, the exercise pressor reflex resets the carotid-vasomotor baroreflex function curve upward on the response arm and rightward to higher arterial pressure; however, it resets the carotid-cardiac curve only rightward to higher arterial pressures (Raven et al. 2006). Although the role of central command and the exercise pressor reflex in resetting of the arterial baroreflex is clear, some recent observations suggest that these neural mechanisms may not be solely responsible for the resetting of the operating point of the reflex during exercise.
Recently, Ogoh et al. (2003b) demonstrated that the carotid-cardiac baroreflex function curve was relocated upward and rightward from rest to heavy dynamic exercise in a workload-dependent manner. However, during low-intensity leg cycling, MAP tended to decrease from rest, and the resetting of the carotid-vasomotor baroreflex function curve was not observed despite exercise-induced increases in central command and exercise pressor reflex activation. A potential explanation for the lack of a blood pressure response and CBR resetting during low-intensity cycling compared to high-intensity cycling could be the powerful influence of the skeletal muscle pump on CBV. Previous studies have indicated that increases in exercise workload decrease the effectiveness of the muscle pump on increasing venous return (Brechue et al. 1995; Rowland & Lisowski, 2001; Sheriff, 2003). The progressive decline in skeletal muscle pump effectiveness with exercise workload could reflect a greater impediment to skeletal muscle blood flow from intramuscular vascular occlusion as compressive forces increase (Brechue et al. 1995). Thus, in contrast to heavy exercise, muscle pump effectiveness is highest during low-intensity exercise, which probably results in an increased venous return and CBV. As a result of the increase in CBV, the cardiopulmonary baroreceptors would be presented with a greater load, which would be expected to decrease sympathetic nerve activity and blood pressure (Saito et al. 1993; Charkoudian et al. 2005, 2006). Thus, the increased cardiopulmonary baroreceptor load may help explain why low-intensity dynamic-cycling exercise (90 b.p.m.) did not change arterial pressure and perhaps more importantly, did not reset the carotid-vasomotor baroreflex function curve (Ogoh et al. 2003b).
Although previous studies have suggested that changes in CBV influence the exercise-induced blood pressure response and the locus of the operating point of the carotid-vasomotor reflex, these experimental paradigms did not account for differences in energy expenditure or exercising heart rates (Volianitis & Secher, 2002; Volianitis et al. 2004). In the present study, we were able to increase CBV and cardiopulmonary baroreceptor load during dynamic exercise while keeping oxygen consumption constant. Importantly, this approach allowed us to have similar exercising heart rates indicating a comparable activation of central command for each of the exercise bouts. Even though we cannot completely rule out differences in exercise pressor reflex activation, particularly during exercise with an increased pedal frequency (i.e. possibly greater mechanoreceptor input), the downward and leftward resetting of the carotid-vasomotor baroreflex function curve suggests this was not the case because one would have expected an augmented upward and rightward resetting if indeed mechanoreceptor input was increased. Thus, by changing posture and pedal frequency we feel we were able to study the influence of increases in CBV and cardiopulmonary baroreceptor load on baroreflex resetting without alterations in energy expenditure or exercising heart rates, thereby minimally altering central command and exercise pressor reflex activation.
The findings of the present study indicate that changes in cardiopulmonary baroreceptor load are able to influence carotid baroreflex resetting during dynamic exercise in humans. Thus, in addition to central command and the exercise pressor reflex, the arterial baroreflex is also being modulated by inputs from cardiopulmonary baroreceptors. From previous studies, we would contend that the feedforward mechanism of central command is the primary regulator of baroreflex resetting, while the feedback mechanism of the exercise pressor reflex is more of a modulator of this resetting (Ogoh et al. 2002; Fadel et al. 2003; Gallagher et al. 2006; Raven et al. 2006). Taking into account the findings of the present study, we now suggest that feedback from the cardiopulmonary baroreceptors also plays a modulatory role in exercise resetting of the arterial baroreflex. Further studies are needed to understand the relative contributions and importance of inputs from the cardiopulmonary baroreceptors in relation to central command and the exercise pressor reflex in determining the degree of baroreflex resetting during exercise.
Postural changes
In the current study, we utilized the supine position to increase CBV during exercise. Indeed, at rest, changing posture from an upright to a supine position increased thoracic admittance and decreased MAP (Table 1). Interestingly, this influence of a postural change on thoracic admittance was maximal at rest. The reason for this is unclear but may be due to leg positioning. Due to the location of the cycle ergometer in front of the subjects, during cycling exercise in the supine position the subjects' legs were slightly elevated while they cycled. We believe this position may have caused maximal loading of cardiopulmonary baroreceptors, and therefore that the arterial pressure response may be different from that in the supine position, in which the legs are straight. Alternatively, it may be that during supine exercise the muscle pump is not very effective in increasing CBV, as indicated by the lack of an increase in thoracic admittance from supine rest. This finding is consistent with previous work indicating that during upright one-legged exercise, the muscle pump increased central venous pressure resulting in a decrease in muscle sympathetic nerve activity from rest via a loading of the cardiopulmonary baroreceptors (Ray et al. 1993). In contrast, during supine one-legged exercise, muscle sympathetic nerve activity did not decrease, probably because the cardiopulmonary baroreceptors were already fully loaded. In addition, (Leyk et al. (1994) suggested that the muscle pump was less effective in the supine position with lower workload. Nevertheless, and of utmost importance to the goals of the current study, thoracic admittance and CBV were still higher during supine exercise compared with control exercise.
Pedal frequency
The increase in pedal frequency during cycle exercise appeared to result in a more effective skeletal muscle pump compared to control exercise as indicated by the significantly greater increase in thoracic admittance (Gotshall et al. 1996; Rowland & Lisowski, 2001; Sheriff, 2003). In an animal model, Sheriff (2003) reported that there is a direct coupling of stride frequency and hindlimb blood flow (i.e. muscle pump). More importantly, Gotshall et al. (1996) measured the cardiovascular responses to increasing pedalling rate (70, 90 and 110 r.p.m.) to assess skeletal muscle pump effectiveness and found that the mean arterial – venous oxygen difference (V˙O2/cardiac output) fell with increasing pedal cadence. This decline in mean arterial – venous oxygen difference indicates that the increased pedalling rate improves the effectiveness of the skeletal muscle pump because of a relative increase in systemic venous return and consequently cardiac output (Gotshall et al. 1996; Rowland & Lisowski, 2001). Nevertheless, several studies have demonstrated that an increase in cycle pedalling rate produces a rise in gross energy expenditure at a constant workload (Gaesser & Brooks, 1975; Seabury et al. 1977; Lollgen et al. 1980; Hagberg et al. 1981; Hagan et al. 1992). The augmented cycling energy cost of an increased cadence is a consequence of factors influencing internal work rather than external work (Hagberg et al. 1981; Wells et al. 1986). Hagan et al. (1992) demonstrated that increases in HR and V˙O2 or blood lactate were higher during cycling exercise at 90 r.p.m. than at 60 r.p.m. for a constant workload. This suggests that increases in cycle pedalling rate may produce greater activation of central command and the exercise pressor reflex, when work rate is kept constant. Therefore, in the present study, all exercise conditions were performed at the same energy expenditure (constant V˙O2) rather than absolute work rate. As a consequence, the exercise workload during 80EX was lower than that during control exercise (44 ± 8 versus 58 ± 9 W; see Table 1). Thus, it could be argued that the lower workload employed during 80EX indicates that central command and exercise pressor reflex activation were reduced, and would explain the attenuated increase in blood pressure. However, known indices of central command such as oxygen uptake and exercising HRs were equivalent, and the rating of perceived exertion reported by the subjects was actually higher during 80EX than control. Taken together, if anything, these markers suggest greater central command activation, and yet the exercise-induced increases in blood pressure and CBR resetting were lower. Thus, we do not feel the slightly lower workload during 80EX explains our findings, and when combined with the supine exercise findings, they provide strong evidence that increases in CBV and cardiopulmonary baroreflex load modulates arterial baroreflex resetting during exercise.
Potential limitations
Although the current experimental protocol was designed to minimize changes in central command and exercise pressor reflex activation, we cannot completely rule out alterations in these neural mechanisms. However, we believe that cycling in the supine position and with a higher pedal frequency provided us with an effective model to study changes in CBV without alterations in oxygen uptake or exercising heart rates, two commonly used indices of central command. In relation to the exercise pressor reflex, even though we cannot completely rule out differences in exercise pressor reflex activation during exercise, it seems reasonable to suggest that any increase in mechanoreceptor input caused by the increased pedal frequency could have been balanced by a decrease in metaboreceptor input due to increased flow and washout of metabolites. However, the degree to which the metaboreflex is activated during low-intensity dynamic exercise is unclear. In this regard, Gallagher et al. (2001a) used 45 Torr lower-body-positive pressure (LBPP) to stimulate the mechanoreceptors, and found that arterial blood pressure was significantly elevated with LBPP from control at rest and throughout exercise with an increase in intramuscular pressure, while HR was unchanged. These data suggested that under normal conditions the mechanoreflex is tonically active and is the primary mediator of exercise pressor reflex-induced alterations in arterial blood pressure during submaximal dynamic exercise in humans.
In relation to the ability of our experimental approach to selectivity unload cardiopulmonary baroreceptors, we realize that alterations in other ongoing reflex mechanisms cannot be completely excluded. However, given the limitations of previous experimental protocols used to alter cardiopulmonary load during exercise (Volianitis & Secher, 2002; Volianitis et al. 2004; Ogoh et al. 2006a,b), the use of changes in posture and pedal frequency provided several advantages including the non-invasiveness and the ability to maintain similar energy expenditures and exercising heart rates. As noted above, we believe the latter limited alterations in central command and exercise pressor reflex activation. Another possibility that cannot be excluded is that changes in CBV directly altered arterial baroreceptors. Indeed, the geometry of the arterial wall has been shown to be substantially modified by alterations in CBV induced by head-up tilting and lower-body negative pressure (Lacolley et al. 1992; Taylor et al. 1995). Although arterial diameters in regions of the arterial baroreceptors were not measured in the current study, we do not believe any such changes altered the carotid or aortic baroreceptors because the maximal gain of the carotid baroreflex function curve was not different between the upright and supine position and there were no differences in pulse pressure between exercise conditions (see Table 1).
Another potential limitation of the study protocol was the use of impedance cardiography to monitor changes in CBV without a measure of central venous pressure. However, we felt the study design only required a marker of a directional change in CBV, and therefore it was unnecessary for subjects to undergo the invasive procedure of a central venous catheter. Furthermore, previous studies have indicated that thoracic admittance provides an accurate measure of changes in CBV in comparison to central venous pressure (Ebert et al. 1986; Pawelczyk et al. 1994; Cai et al. 2000). Pawelczyk et al. (1994) reported a strong relationship between changes in thoracic impedance and central blood volume during head-up tilt. Estimating changes in CBV from upright rest to exercise by applying the equation from the work of Pawelczyk and colleagues (1994) to the impedance data of the present study, we derived CBV values of Δ = 135 ± 26 ml, Δ = 184 ± 28 ml, and Δ = 174 ± 20 ml for the change during control, SupEX and 80EX conditions, respectively. Although it is unclear if this equation is valid for use to approximate CBV during exercise, these calculations demonstrate the expected changes reported and provide the reader with an estimate of actual changes in CBV for reference.
In the present study it is plausible that the rapid NP and NS protocol may have underestimated carotid baroreflex function due to a counteracting influence of the aortic baroreceptors in response to the change in arterial pressure induced by NP and NS. However, by limiting this manoeuvre to 12–15 s, we would contend that any counteraction from the aortic baroreflex would be minimal. This contention is based on previous work demonstrating generally well-maintained CBR-mediated responses with the application of either sustained or pulsatile NP and NS (Mancia et al. 1978, 1985; Ogoh et al. 2003a). Also, numerous studies have indicated similar CBR-mediated responses at rest and during exercise using the rapid pulse train technique, without any indication of counteraction of the CBR-mediated responses (Ogoh et al. 2002; Fadel et al. 2003; Fisher et al. 2006; Gallagher et al. 2006).
In summary, by changing posture and pedal frequency we were able to study the influence of increases in CBV and cardiopulmonary baroreceptor load on baroreflex resetting during dynamic exercise. Importantly, we were able to match energy expenditure and exercising heart rates, thereby minimally altering central command and exercise pressor reflex activation. Our results indicate that the carotid-vasomotor baroreflex function curve was relocated downward and leftward, and blood pressure was lower when CBV was elevated. Thus, increasing CBV and loading the cardiopulmonary baroreceptors reduces the magnitude of the exercise-induced increases in MAP and CBR resetting. Collectively, these data suggest that the degree of arterial baroreflex resetting during exercise is influenced not only by central command and the exercise pressor reflex, but also by inputs from the receptor populations sensing CBV which comprise the cardiopulmonary baroreflex.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO, USA. The authors appreciate the time and effort expended by all the volunteer subjects. This study was supported in part by American College of Sports Medicine Visiting Scholar Award, American Heart Association Grant No. 0465104Y and NIH Grant No. HL045547.
References
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Brechue WF, Ameredes BT, Barclay JK, Stainsby WN. Blood flow and pressure relationships which determine V˙O2max. Med Sci Sports Exerc. 1995;27:37–42. [PubMed] [Google Scholar]
- Cai Y, Holm S, Jenstrup M, Stromstad M, Eigtved A, Warberg J, Hojgaard L, Friberg L, Secher NH. Electrical admittance for filling of the heart during lower body negative pressure in humans. J Appl Physiol. 2000;89:1569–1576. doi: 10.1152/jappl.2000.89.4.1569. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol. 2006;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK. The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med. 1986;57:49–53. [PubMed] [Google Scholar]
- Eckberg DL. Baroreflex inhibition of the human sinus node: importance of stimulus intensity, duration, and rate of pressure change. J Physiol. 1977;269:561–577. doi: 10.1113/jphysiol.1977.sp011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol. 2003;88:671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Dawson EA, Fadel PJ, Secher NH, Raven PB, White MJ. Cardiac and vasomotor components of the carotid baroreflex control of arterial blood pressure during isometric exercise in humans. J Physiol. 2006;572:869–880. doi: 10.1113/jphysiol.2005.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol. 1975;38:1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Smith SA, Norton KH, Querry RG, Olivencia-Yurvati A, Raven PB. Increases in intramuscular pressure raise arterial blood pressure during dynamic exercise. J Appl Physiol. 2001a;91:2351–2358. doi: 10.1152/jappl.2001.91.5.2351. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Smith SA, Stromstad M, Ide K, Secher NH, Raven PB. The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp Physiol. 2006;91:79–87. doi: 10.1113/expphysiol.2005.032110. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol. 2001b;533:871–880. doi: 10.1111/j.1469-7793.2001.t01-2-00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol. 2001c;533:861–870. doi: 10.1111/j.1469-7793.2001.t01-1-00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotshall RW, Bauer TA, Fahrner SL. Cycling cadence alters exercise hemodynamics. Int J Sports Med. 1996;17:17–21. doi: 10.1055/s-2007-972802. [DOI] [PubMed] [Google Scholar]
- Hagan RD, Weis SE, Raven PB. Effect of pedal rate on cardiorespiratory responses during continuous exercise. Med Sci Sports Exerc. 1992;24:1088–1095. [PubMed] [Google Scholar]
- Hagberg JM, Mullin JP, Giese MD, Spitznagel E. Effect of pedaling rate on submaximal exercise responses of competitive cyclists. J Appl Physiol. 1981;51:447–451. doi: 10.1152/jappl.1981.51.2.447. [DOI] [PubMed] [Google Scholar]
- Idema RN, Van Den Meiracker AH, Imholz BP, Man In ‘T, Veld AJ, Settels JJ, Ritsema Van Eck HJ, Schalekamp MA. Comparison of Finapres non-invasive beat-to-beat finger blood pressure with intrabrachial artery pressure during and after bicycle ergometry. J Hypertens. 1989;7(Suppl):S58–S59. doi: 10.1097/00004872-198900076-00025. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Raimondi G, Peruzzi G. Baroreflex control of sinus node during dynamic exercise in humans: effects of central command and muscle reflexes. Am J Physiol Heart Circ Physiol. 1997;272:H1157–H1164. doi: 10.1152/ajpheart.1997.272.3.H1157. [DOI] [PubMed] [Google Scholar]
- Imholz BP. Automated blood pressure measurement during ergometric stress testing: possibilities of Finapres. Z Kardiol. 1996;85(Suppl. 3):76–80. [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Lacolley PJ, Pannier BM, Slama MA, Cuche JL, Hoeks AP, Laurent S, London GM, Safar ME. Carotid arterial haemodynamics after mild degrees of lower-body negative pressure in man. Clin Sci. 1992;83:535–540. doi: 10.1042/cs0830535. [DOI] [PubMed] [Google Scholar]
- Leyk D, Essfeld D, Hoffmann U, Wunderlich HG, Baum K, Stegemann J. Postural effect on cardiac output, oxygen uptake and lactate during cycle exercise of varying intensity. Eur J Appl Physiol Occup Physiol. 1994;68:30–35. doi: 10.1007/BF00599238. [DOI] [PubMed] [Google Scholar]
- Lollgen H, Graham T, Sjogaard G. Muscle metabolites, force, and perceived exertion bicycling at varying pedal rates. Med Sci Sports Exerc. 1980;12:345–351. [PubMed] [Google Scholar]
- McDowall LM, Dampney RA. Calculation of threshold and saturation points of sigmoidal baroreflex function curves. Am J Physiol Heart Circ Physiol. 2006;291:H2003–H2007. doi: 10.1152/ajpheart.00219.2006. [DOI] [PubMed] [Google Scholar]
- McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1454–H1463. doi: 10.1152/ajpheart.2001.280.4.H1454. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G, Ferrari A, Zanchetti A. Reflex cardiovascular regulation in humans. J Cardiovasc Pharmacol. 1985;7(Suppl. 3):S152–S159. doi: 10.1097/00005344-198500073-00018. [DOI] [PubMed] [Google Scholar]
- Mancia G, Ludbrook J, Ferrari A, Gregorini L, Zanchetti A. Baroreceptor reflexes in human hypertension. Circ Res. 1978;43:170–177. doi: 10.1161/01.res.43.2.170. [DOI] [PubMed] [Google Scholar]
- Norton KH, Boushel R, Strange S, Saltin B, Raven PB. Resetting of the carotid arterial baroreflex during dynamic exercise in humans. J Appl Physiol. 1999;87:332–338. doi: 10.1152/jappl.1999.87.1.332. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB. Cardiopulmonary baroreflex is reset during dynamic exercise. J Appl Physiol. 2006a;100:51–59. doi: 10.1152/japplphysiol.00804.2005. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha SA, Raven PB. Effects of changes in central blood volume on carotid-vasomotor baroreflex sensitivity at rest and during exercise. J Appl Physiol. 2006b;101:68–75. doi: 10.1152/japplphysiol.01452.2005. OY. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Hardisty JM, Wasmund WL, Keller DM, Raven PB, Smith ML. Does pulsatile and sustained neck pressure or neck suction produce differential cardiovascular and sympathetic responses in humans? Exp Physiol. 2003a;88:595–601. doi: 10.1113/eph8802586. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol. 2003b;550:317–324. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol. 2002;543:349–364. doi: 10.1113/jphysiol.2002.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Gauthier JP, Rowell LB. Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J Appl Physiol. 1994;77:502–506. doi: 10.1152/jappl.1994.77.2.502. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Matzen S, Friednai DB, Secher NH. Cardiovascular and hormonal response to central hypovolaemia in humans. In: Secher NH, Pawelczyk JA, Ludbrook J, editors. Blood Loss and Shock. 1. Boston: Little Brown; 1994. pp. 25–36. [Google Scholar]
- Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol Heart Circ Physiol. 1989;257:H1389–H1395. doi: 10.1152/ajpheart.1989.257.5.H1389. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 1993;265:H1928–H1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Querry RG, Smith SA, Stromstad M, Ide K, Raven PB, Secher NH. Neural blockade during exercise augments central command's contribution to carotid baroreflex resetting. Am J Physiol Heart Circ Physiol. 2001;280:H1635–H1644. doi: 10.1152/ajpheart.2001.280.4.H1635. [DOI] [PubMed] [Google Scholar]
- Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol Heart Circ Physiol. 1993;264:H1–H7. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Rowland T, Lisowski R. Hemodynamic responses to increasing cycle cadence in 11-year old boys: role of the skeletal muscle pump. Int J Sports Med. 2001;22:405–409. doi: 10.1055/s-2001-16245. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Seabury JJ, Adams WC, Ramey MR. Influence of pedalling rate and power output on energy expenditure during bicycle ergometry. Ergonomics. 1977;20:491–498. doi: 10.1080/00140137708931658. [DOI] [PubMed] [Google Scholar]
- Sheriff DD. Muscle pump function during locomotion: mechanical coupling of stride frequency and muscle blood flow. Am J Physiol Heart Circ Physiol. 2003;284:H2185–H2191. doi: 10.1152/ajpheart.01133.2002. [DOI] [PubMed] [Google Scholar]
- Shi X, Andresen JM, Potts JT, Foresman BH, Stern SA, Raven PB. Aortic baroreflex control of heart rate during hypertensive stimuli: effect of fitness. J Appl Physiol. 1993;74:1555–1562. doi: 10.1152/jappl.1993.74.4.1555. [DOI] [PubMed] [Google Scholar]
- Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol. 2003;551:1013–1021. doi: 10.1113/jphysiol.2003.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. ‘Non-hypotensive’ hypovolaemia reduces ascending aortic dimensions in humans. J Physiol. 1995;483:289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544:977–984. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianitis S, Yoshiga CC, Vogelsang T, Secher NH. Arterial blood pressure and carotid baroreflex function during arm and combined arm and leg exercise in humans. Acta Physiol Scand. 2004;181:289–295. doi: 10.1111/j.1365-201X.2004.01292.x. [DOI] [PubMed] [Google Scholar]
- Wells R, Morrissey M, Hughson R. Internal work and physiological responses during concentric and eccentric cycle ergometry. Eur J Appl Physiol Occup Physiol. 1986;55:295–301. doi: 10.1007/BF02343802. [DOI] [PubMed] [Google Scholar]