Abstract
This study examines the molecular basis for the T-type and L-type Ca2+ currents in canine Purkinje cells. The ICaT in Purkinje cells was completely suppressed by 200 nm kurtoxin, a specific blocker of both Cav3.1 and Cav3.2 channels. Since only Cav3.2 mRNA is expressed at high levels in Purkinje fibres, being approximately 100-fold more abundant than either Cav3.1 or Cav3.3 mRNAs, it is concluded that the Cav3.2 gene encodes the bulk of the T-type Ca2+ channels in canine Purkinje cells. This conclusion is consistent with the sensitivity of the current to blockade by Ni2+ ions (KD = 32 μm). For L-type channels, Cav1.2 mRNA was most abundant in Purkinje fibres but a significant level of Cav1.3 mRNA expression was also found. A comparison of the sensitivity to blockade by isradipine of the L-type currents in Purkinje cells and ventricular epicardial myocytes, which only express Cav1.2, suggests that the Cav1.3 channels make, at most, a minor contribution to the L-type current in canine Purkinje cells.
Two types of calcium currents have been described in canine Purkinje cells: L-type (ICaL) and T-type (ICaT) (Hirano et al. 1989; Tseng & Boyden, 1989). The L-type Ca2+ current is ubiquitous in cardiac myocytes. In contrast, the T-type calcium current is expressed in a more restricted pattern in the heart, being found mainly in Purkinje cells, sinus node cells and embryonic myocytes (reviewed in Vassort et al. 2006), although it has been described at low levels in adult ventricular myocytes in some species (Mitra & Morad, 1986; Tseng & Boyden, 1989; Wang & Cohen, 2003). This expression pattern, together with the biophysical properties of the T-type Ca2+ channels, is consistent with a role for these channels in conduction and pacemaking (Vassort et al. 2006).
Three different subfamilies of genes encoding Ca2+ channel principal subunits have been described (Catterall et al. 2005). Members of the first subfamily (Cav1 genes) encode channels conducting typical L-type currents. In cardiac myocytes, the bulk of ICaL is conducted by channels encoded by the Cav1.2 gene (CACNA1C or α1C) (Striessnig, 1999). The Cav1.3 (CACNA1D or α1D) channel may contribute to the L-type currents in atrioventricular and sinus node cells (Bohn et al. 2000; Platzer et al. 2000; Mangoni et al. 2003). The other two members of the Cav1 family are not significantly expressed in the myocardium (Striessnig, 1999; Catterall et al. 2005).
The second subfamily of Ca2+ channel principal subunit genes (Cav2) encodes channels conducting currents whose biophysical properties are intermediate between L- and T-type currents. These genes are the molecular correlates of the N-, P/Q- and R- types of Ca2+ currents (Catterall et al. 2005). Although some of these genes have been reported to be expressed in the heart (reviewed in Catterall et al. 2005), no corresponding currents have been described in normal cardiac myocytes to date.
Three genes are found in the third subfamily: Cav3.1 (CACNA1G or α1G), Cav3.2 (CACNA1H or α1H) and Cav3.3 (CACNA1I or α1I) (Perez-Reyes et al. 1998; Cribbs et al. 1998; Lee et al. 1999a; Catterall et al. 2005). The biophysical properties of the encoded channels are those of typical T-type Ca2+ currents. Cav3 genes have been reported to be expressed in sinus node cells (Bohn et al. 2000), embryonic hearts (Niwa et al. 2004) and Purkinje cells (Han et al. 2002).
Recent studies have investigated ion channel gene expression in Purkinje fibres (Shi et al. 1999; Han et al. 2002; Pourrier et al. 2003), but a detailed analysis of the molecular basis of Ca2+ currents in these cells has not been performed. In this paper, we examine the molecular basis of the canine Purkinje fibre T- and L-type calcium currents.
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee of Stony Brook University and Columbia University.
Isolation of RNA from Canine Left Ventricular Wall and Purkinje Fibres
Adult cross-breed dogs were killed with pentobarbital (80 mg kg−1, i.v., via the cephalic vein). Free-running Purkinje fibres or portions of the left ventricular free wall were dissected and quick-frozen in liquid nitrogen. Total RNA was prepared using RNeasy columns (Qiagen). A DNase I treatment step was included to eliminate genomic DNA contamination.
Real-time PCR Analysis
Quantification of mRNA for the calcium channel genes was performed using real-time PCR analysis, as previously described (Rosati et al. 2006). Variability between samples was assessed by measuring 18S and 28S internal control genes. Multiple primer pairs were used for gene-specific amplification. This approach reveals any potential heterogeneity in amplification efficiencies for the different primer pairs, making the comparison of the expression levels of different genes more accurate.
Primer sequences were as follows
18S
Forward(1): CCTGCGGCTTAATTTGACTC, Reverse(1): CGGACATCTAAGGGCATCAC; Forward(2): GTTCCGACCATAAACGATGC, Reverse(2): AACTAAGAACGGCCATGCAC; Forward(3): CCCGAAGCTTTACTTTGAA, Reverse(3): GCATCGTTTATGGTCGGAAC.
28S
Forward(1): TGGGTTTTAAGCAGGAGGTG, Reverse(1): TCCTCAGCCAAGCACATACA; Forward(2): AAAGGGAGTCGGGTTCAGAT, Reverse(2): AGAGGCTGTTCACCTTGGAG; Forward(3): TCCGAAGTTTCCCTCAGGAT, Reverse(3): CCTTTTCTGGGGTCTGATGA.
Cav1.1
Forward(1): ACCGGAACAACAACTTCCAG, Reverse(1): ATGGCCTTGAACTCATCCAG; Forward(2): TCATTCTGCTGGGATCCTTC, Reverse(2): CCTCCAGTGGCGGATGAACT.
Cav1.2
Forward(1): CGATTTTTGCCAATTGTGTG, Reverse(1): CTCAGTGCCTTCACATCGAA; Forward(2): ATGAAGGCATGGATGAGGAG, Reverse(2): CCAGTGGGGCTGATTGTAGT.
Cav1.3
Forward(1): GAACAAGGACTGGTGGGAAA, Reverse(1): GAACTGAAGGCCTTTGGACA; Forward(2): TATGATGGCGCTCTTCACGG, Reverse(2): GTAGGGGTTTTTGGGGATGT.
Cav1.4
Forward(1): CCAAGAACCCACATCAATAT, Reverse(1): TTCAGTGACGGCAATATCCA.
Cav3.1
Forward(1): CAGGCAGCAATAAGGACTGA, Reverse(1): GGAGGTCTCCTGAAATCCAG; Forward(2): CATCTCTTTGGCTGCAAATT, Reverse(2): ACTGTCCACTCGCATCTTCC; Forward(3): CATCAGCATGTTGGTCATCC, Reverse(3): GAAGCTGACGTTCTGCAGGT.
Cav3.2
Forward(1): GTCAGCCACATCACCAGCTC, Reverse(1): GGGGGGCTCATCTTCTTCTT; Forward(2): GCTGCAAGTTCAGTCTGACG, Reverse(2): CTCCAAGTGGGTGGATGTCT; Forward(3): TCAACGTCATCACCATGTCC, Reverse(3): CTTGAGCAGCTTCAGAACTC.
Cav3.3
Forward(1): TCTTCAAGGACCGATGGAAC, Reverse(1): CAGACCAGCTTCCCAAAGAG; Forward(2): CATCTTTGGCTGCAAATTCA, Reverse(2): TTGGATGAGCTCTGGTCCTC; Forward(3): GCCAAGGACGTCTTTACCAA, Reverse(3): AGGAAGATGCGTTCAGTGCT.
For each gene, results obtained from the different sets of primer pairs were very consistent. All of the primer pairs were first tested using canine brain mRNA as a positive control. The specificity of the amplified products was determined by gel electrophoresis and sequence analysis.
All of the primers were targeted to amplify regions devoid of alternative splicing. When no information on splice variants for a given gene was available in the canine, we used information available for the corresponding human gene. In all of the reported experiments only a single amplification product was detected.
Electrophysiological recordings
Dissociation and recording techniques have been previously described (Tseng & Boyden, 1989). Patch pipettes were filled with an internal solution that had the following composition (mm): CsOH 125, aspartic acid 125, tetraethylammonium chloride 20, Hepes 10, Mg-ATP 5, EGTA 10 and phosphocreatine 3.6 (pH 7.3 with CsOH). A period of 5–10 min was then allowed for intracellular dialysis to begin before switching to a nominal sodium-free extracellular recording solution (mm): CaCl2 5.0, MgCl2 0.5, tetraethylammonium chloride 140, Hepes 12, dextrose 10, and 4-aminopyridine 2 (pH 7.3 with CsOH). After the appropriate controls, all recordings were performed in the presence of 20 μm tetrodotoxin (TTX) to avoid contamination of the currents with the TTX-sensitive Ca2+ current (ICaTTX) (Lemaire et al. 1995; Cole et al. 1997).
To separate the T- and L-type Ca2+ currents, different holding voltages were used. Typically, a holding voltage (Vh) of −40 mV (for the L-type current) and −70 or −90 mV (for the T-type current) was used. In preliminary experiments, we found negligible inactivation of the T-current at the holding voltages specified above. The T-type current was isolated from the L-type current by subtracting the current recorded at a Vh of −40 mV from that recorded at the more negative Vh values. TTX (Alomone Laboratories, Jerusalem, Israel or Sigma, St Louis, MO, USA), ω-conotoxin MVIIC (Alomone Laboratories), isradipine (Sigma) NiCL2 (Fisher Scientific) and kurtoxin (Sigma) were added fresh on the day of the experiment.
To calculate the KD for nickel ion blockade of ICaT, data points were fitted with the Hill equation: fractional inhibition = 1/(1 + (KD/[Ni2+])).
Results
Calcium currents in canine Purkinje cells
Two primary components can be observed in recordings of total calcium currents from isolated canine Purkinje cells (Fig. 1). These two components correspond to the T-type and L-type calcium currents and have been previously described (Tseng & Boyden, 1989; Hirano et al. 1989). The currents can be distinguished on the basis of different thresholds for activation, steady-state inactivation curves and time constants of inactivation.
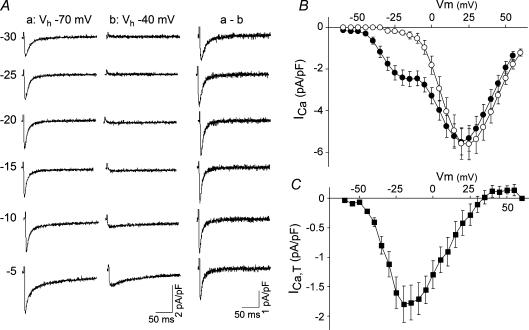
Figure 1. L- and T-type calcium currents in canine Purkinje cells.
A, recordings of total calcium currents elicited in an isolated Purkinje cell in response to depolarizing voltage steps (values on the left of the corresponding traces), from holding potentials (Vh) of −70 (left column) or −40 mV (centre column), as indicated. Difference currents for each test pulse are shown in the right column (a – b). B, current–voltage relationship for the total calcium current elicited from Vh of either −70 mV (•, n = 10 cells, mean ± s.e.m.) or −40 mV (○, n = 7 cells, mean ± s.e.m.). C, current–voltage relationship of the T-type current, as isolated by subtraction of the total current at Vh = −40 mV from the current recorded at Vh = −70 mV. Mean data ± s.e.m. (n = 7) are shown.
In Purkinje cells, the threshold for activation of the T-type current is relatively positive compared to the T-type currents found in many other cells, being approximately −45 mV. Average values for the current density for the T-type and L-type currents in Purkinje cells are 1.8 ± 0.3 pA pF−1 (mean ±s.e.m., n = 7) and 5.5 ± 0.6 pA pF−1 (n = 10), respectively.
Toxin sensitivity of the T-type calcium current
The T-type current found in Purkinje cells could, in principle, be encoded by members of either the Cav3 or Cav2 family of calcium channel genes, because the activation threshold of the current is located at a relatively hyperpolarized membrane potential. To address this issue, we examined the sensitivity of the T-type current to two toxins that are specific blockers of channels encoded by either the Cav2 gene family or the Cav3 gene family.
ω-Conotoxin MVIIC (ω-CTX) is a specific blocker of the Cav2.1 (Sather et al. 1993) and Cav2.2 (Stocker et al. 1997) channels, as well as of the native N-type and P/Q-type Ca2+ currents (McDonough et al. 1996). Application of 1 μm ω-CTX did not result in a reduction of the peak T-type calcium current (not shown).
Kurtoxin is a potent and specific blocker of Cav3.1 and Cav3.2 channels that does not affect Cav2.1, Cav2.2 or Cav2.3 currents (Chuang et al. 1998). Application of this toxin results in an almost complete elimination of the low threshold component of the total calcium current (Fig. 2A–C), ruling out a major involvement of any of the Cav2 channel genes in ICaT and indicating that the Cav3 genes underlie the T-type calcium current in canine Purkinje cells.
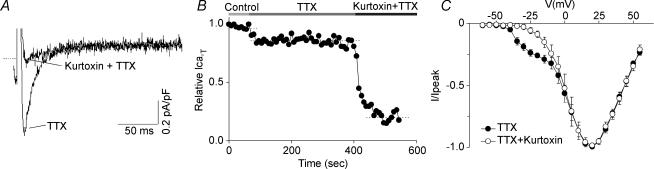
Figure 2. Effect of kurtoxin on the T-type calcium current in canine Purkinje cells.
A, kurtoxin selectively blocks the T-type component of the calcium current in Purkinje cells. The current was elicited by a test pulse at −25 mV from a holding potential of −90 mV, in control conditions and after the application of 200 nm kurtoxin, as indicated. Both the control and drug recordings were performed in the presence of 20 μm TTX (see Methods for rationale). B, time course for the T-type calcium current inhibition by kurtoxin. The horizontal bars on top of the graph indicate the bath solution applied. C, average current–voltage plots obtained in n = 3 cells under control conditions and after application of kurtoxin (see legend). The holding potential was −70 mV and test pulses were applied from −60 to +55 mV at 5 mV intervals. Error bars represent the s.e.m.
Quantitative comparison of CaV3 subfamily mRNA expression in Purkinje fibres
Based on the effects of kurtoxin and ω-CTX, it seemed likely that the T-type current in Purkinje cells was encoded by one or possibly both of the Cav3.1 and Cav3.2 genes. Therefore the relative mRNA expression of the Cav3 genes in Purkinje fibres was examined using real-time PCR.
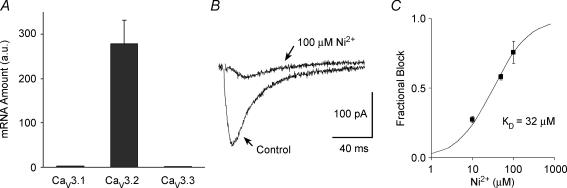
As seen in Fig. 3A, only the Cav3.2 gene is expressed at significant levels in Purkinje fibres, being 100-fold more abundant than the Cav3.1 and Cav3.3 genes. This result, in combination with kurtoxin sensitivity, strongly suggests that the Cav3.2 channel makes the primary contribution to the T-type current in Purkinje cells.
Figure 3. Molecular basis of the T-type calcium current in canine Purkinje cells.
A, bar graph of relative mRNA abundance for the Cav3.1, Cav3.2 and Cav3.3 genes in Purkinje fibres. The mRNA quantities were determined by real-time PCR and are represented in arbitrary units. Data are means ± s.e.m. (n = 4, each sample from 3 to 6 different hearts). B, sample recording of ICaT in a canine Purkinje cell, in control conditions (see Methods) and in the presence of 100 μm Ni2+, as indicated by the arrows. The current was elicited by a −30 mV test pulse from a holding potential of −90 mV. C, dose–response curve for Ni2+ blockade of ICaT. The fractional block of the ICaT is shown for three different Ni2+ concentrations: 10, 50 and 100 μm. Data points are average values obtained in n = 3 cells from 2 different hearts. Data points were fitted with the Hill equation (see Methods), yielding a KD of 32 ± 3 μm (mean ± s.e.m.).
It has been shown that the Ni2+ sensitivity of Cav3.2 channels is about 20-fold higher than for Cav3.1 channels (Lee et al. 1999b). Dose–response analysis for Ni2+ blockade of ICaT in canine Purkinje cells yielded a KD value for channel blockade of 32 ± 3 μm (Fig. 3C), indicating that this current is highly sensitive to Ni2+ ions (Fig. 3B) and confirming the primary contribution of the Cav3.2 channel to the native current.
Quantitative comparison of CaV1 subfamily mRNA expression in canine ventricle
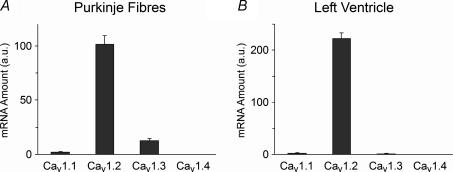
The L-type calcium current in Purkinje cells has the typical properties of L-type channels encoded by the Cav1 subfamily of calcium channel genes (Hirano et al. 1989; Tseng & Boyden, 1989). To determine the molecular basis of the L-type calcium current in Purkinje cells, mRNA expression from the four known Cav1 genes was examined. In Purkinje fibres, Cav1.2 mRNA is the most abundant Cav1 gene subfamily mRNA (Fig. 4A). There is, however, a significant level of Cav1.3 mRNA expression.
Figure 4. Cav1 family gene expression in the canine Purkinje fibres and left ventricle.
Bar graph of relative mRNA abundance for the Cav1.1, Cav1.2, Cav1.3 and Cav1.4 genes in Purkinje fibres (A) and left ventricle (B). The mRNA quantities were determined by real-time PCR and are represented in arbitrary units. Data are means ± s.e.m. (n = 3–5, each Purkinje sample from 3 to 6 hearts; each ventricular sample from 1 heart).
In relative terms, Cav1.2 mRNA comprises 87% of the total Cav1 mRNA in Purkinje fibres, with Cav1.3 mRNA making up most of the remainder (11% of total). This differs from ventricular myocytes, where Cav1.2 mRNA comprises 98% of the total Cav1 mRNA and Cav1.3 contributes only 1% of the total (Fig. 4B).
The Cav1.3 channel is less sensitive to block by dihydropyridines (DHP) than the Cav1.2 channel (Xu & Lipscombe, 2001; Koschak et al. 2001). In order to test whether Cav1.3 channels make a significant functional contribution to the L-type calcium current in Purkinje cells, we compared the sensitivity of Purkinje cell and ventricular myocyte L-type currents to the DHP compound isradipine. There was no significant difference in isradipine sensitivity of ICaL in Purkinje cells versus epicardial myocytes. The percentage inhibition of ICaL by 300 nm isradipine was 46.7 ± 9.9 (n = 6) in Purkinje cells and 40.4 ± 5.7 (n = 5) in epicardial myocytes (mean ± s.e.m.).
Discussion
The presence of two types of Ca2+ currents, L-type and T-type, in canine Purkinje fibres has been reported previously (Hirano et al. 1989; Tseng & Boyden, 1989). In keeping with these prior observations, in all cells tested a large ICaT was detected in addition to ICaL.
Three genes are known to encode ICaT α subunits, namely Cav3.1, Cav3.2 and Cav3.3. Other channels, though, have been shown to produce T-type currents under certain conditions. In particular, Cav2.1 (α1B), Cav2.3 (α1E) and Cav1.2 (α1C) can produce T-type-like channels in the absence of auxiliary subunits (Meir & Dolphin, 1998). Moreover, Cav1.3 (α1D) has been shown to produce currents endowed with a more negative activation threshold and more rapid kinetics when compared to classic L-type currents (Lipscombe et al. 2004). Since subtle changes in the biophysical properties of the currents could be due to uncharacterized differences in accessory subunit composition or splice variants, we used pharmacological tools to narrow down the set of genes that could potentially encode the Purkinje cell ICaT α subunit. The sensitivity of Purkinje cell ICaT to kurtoxin, combined with the failure of ω-conotoxin MVIIC to block the current, indicates that the α subunit belongs to the Cav3 family.
The three Cav3 channels can be distinguished on the basis of their sensitivity to blockade by Ni2+ ions. Lee et al. (1999b) have shown that recombinant Cav3.2 currents are blocked by relatively low Ni2+ concentrations (IC50 = 13 μm), while Cav3.3 and Cav3.1 currents are much less sensitive to blockade with this cation, with IC50 values nearly 20-fold higher (IC50 = 216 and 250 μm, respectively). We and others have previously shown that ICaT in canine Purkinje cells is highly sensitive to Ni2+ ions (Hirano et al. 1989; Tseng & Boyden, 1989). In this report, we determined the KD for Ni2+ blockade of ICaT to be 32 μm, which is close to the IC50 for the block of the recombinant Cav3.2 channels, given the difficulty in recording the native current completely uncontaminated by L-type current. This result is consistent with the observation that Cav3.2 mRNA is two orders of magnitude more abundant than either Cav3.1 or Cav3.3 mRNA transcripts in Purkinje fibres and supports the hypothesis that Cav3.2 channels comprise the bulk of ICaT in canine Purkinje fibres.
At this time, reliable antibodies against the Cav3 ion channel proteins are unavailable and expression analysis for these channels was therefore limited to mRNA. Nonetheless, the very large difference in gene expression among the Cav3 genes (nearly two orders of magnitude) suggests that a qualitative discordance between mRNA and protein expression for the Cav3 channels is unlikely.
Our Cav3 gene expression data in Purkinje fibres (Fig. 3A) are at variance with an earlier report (Han et al. 2002). There are several potential problems that can arise with the application of PCR for mRNA quantification (Nolan et al. 2006) and it is likely that the discordance of the results resides in technical differences between the two studies. Han et al. (2002) used competitive PCR, whereas real-time PCR was used in this report. More importantly, multiple-primer pairs were used in the current study to detect potential variation in amplification efficiency for different primer pairs, whereas only a single primer pair was used by Han et al. (2002). In addition, all reaction products from the experiments described in this report were sequenced to confirm that the correct amplicon was obtained. While we do not have a definitive explanation for this discrepancy, the molecular data reported here are entirely consistent with the pharmacology of the ICaT in canine Purkinje cells.
In ventricular myocytes, ICaL is encoded by the Cav1.2 gene. This gene is also the most abundantly expressed member of the Cav1 subfamily in canine Purkinje fibres (Fig. 4A). Cav1.3 mRNA is also expressed at significant levels in canine Purkinje fibres, suggesting a possible contribution of this channel to ICaL in Purkinje cells. To test this possibility, we compared the sensitivity of the L-type current in Purkinje cells and ventricular myocytes to isradipine. Since the Cav1.2 channel is more sensitive to dihydropyridine blockade than the Cav1.3 channel (Xu & Lipscombe, 2001; Koschak et al. 2001; Lipscombe et al. 2004), a significant contribution of the latter gene to the ICaL would be expected to result in a lower sensitivity of the current to isradipine. The results indicate that there is no significant difference in the isradipine sensitivity of ICaL in the two tissues. Although this assay has limited sensitivity, it is reasonable to conclude that the Cav1.3 channel does not contribute to the ICaL in canine Purkinje cells out of proportion to the relative abundance of its cognate mRNA.
Taken together, the pharmacology and gene expression data support the conclusion that the primary component of the T-type calcium current in canine Purkinje cells is encoded by the Cav3.2 gene and the Purkinje cell L-type current is encoded primarily by the Cav1.2 gene.
Acknowledgments
The authors wish to thank Mrs. Lldia Colzi Rosati and Ms. Emma G. McKinnon for their help with this project. This work was supported by NIH grants HL-28958 (D.M.), NS-29755 (D.M.) and HL 58860 (P.B.) and AHA grant 0235467T (B.R.). The project was facilitated by Dr Ira Cohen’s support and Joan Zuckerman’s skilled technical assistance.
References
- Bohn G, Moosmang S, Conrad H, Ludwig A, Hofmann F, Klugbauer N. Expression of T- and L-type calcium channel mRNA in murine sinoatrial node. FEBS Lett. 2000;481:73–76. doi: 10.1016/s0014-5793(00)01979-7. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chuang RS, Jaffe H, Cribbs L, Perez-Reyes E, Swartz KJ. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat Neurosci. 1998;1:668–674. doi: 10.1038/3669. [DOI] [PubMed] [Google Scholar]
- Cole WC, Chartier D, Martin M, Leblanc N. Ca2+ permeation through Na+ channels in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol. 1997;273:H128–H137. doi: 10.1152/ajpheart.1997.273.1.H128. [DOI] [PubMed] [Google Scholar]
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- Han W, Bao W, Wang Z, Nattel S. Comparison of ion-channel subunit expression in canine cardiac Purkinje fibers and ventricular muscle. Circ Res. 2002;91:790–797. doi: 10.1161/01.res.0000039534.18114.d9. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Fozzard HA, January CT. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol Heart Circ Physiol. 1989;256:H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999 a;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J. 1999 b;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire S, Piot C, Seguin J, Nargeot J, Richard S. Tetrodotoxin-sensitive Ca2+ and Ba2+ currents in human atrial cells. Receptors Channels. 1995;3:71–81. [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Swartz KJ, Mintz IM, Boland LM, Bean BP. Inhibition of calcium channels in rat central and peripheral neurons by ω-conotoxin MVIIC. J Neurosci. 1996;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir A, Dolphin AC. Known calcium channel α1 subunits can form low threshold small conductance channels with similarities to native T-type channels. Neuron. 1998;20:341–351. doi: 10.1016/s0896-6273(00)80461-4. [DOI] [PubMed] [Google Scholar]
- Mitra R, Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986;83:5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa N, Yasui K, Opthof T, Takemura H, Shimizu A, Horiba M, Lee JK, Honjo H, Kamiya K, Kodama I. Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am J Physiol Heart Circ Physiol. 2004;286:H2257–H2263. doi: 10.1152/ajpheart.01043.2003. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nature Protocols. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pourrier M, Zicha S, Ehrlich J, Han W, Nattel S. Canine ventricular KCNE2 expression resides predominantly in Purkinje fibers. Circ Res. 2003;93:189–191. doi: 10.1161/01.RES.0000084851.60947.B5. [DOI] [PubMed] [Google Scholar]
- Rosati B, Grau F, McKinnon D. Regional variation in mRNA transcript abundance within the ventricular wall. J Mol Cell Cardiol. 2006;40:295–302. doi: 10.1016/j.yjmcc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sather WA, Tanabe T, Zhang JF, Mori Y, Adams ME, Tsien RW. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel α1 subunits. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res. 1999;85:e1–6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- Stocker JW, Nadasdi L, Aldrich RW, Tsien RW. Preferential interaction of ω-conotoxins with inactivated N-type Ca2+ channels. J Neurosci. 1997;17:3002–3013. doi: 10.1523/JNEUROSCI.17-09-03002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J. Pharmacology, structure and function of cardiac L-type Ca2+ channels. Cell Physiol Biochem. 1999;9:242–269. doi: 10.1159/000016320. [DOI] [PubMed] [Google Scholar]
- Tseng GN, Boyden PA. Multiple types of Ca2+ currents in single canine Purkinje cells. Circ Res. 1989;65:1735–1750. doi: 10.1161/01.res.65.6.1735. [DOI] [PubMed] [Google Scholar]
- Vassort G, Talavera K, Alvarez JL. Role of T-type Ca2+ channels in the heart. Cell Calcium. 2006;40:205–220. doi: 10.1016/j.ceca.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Wang HS, Cohen IS. Calcium channel heterogeneity in canine left ventricular myocytes. J Physiol. 2003;547:825–833. doi: 10.1113/jphysiol.2002.035410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Cav1.3α1, L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]