Figure 1.
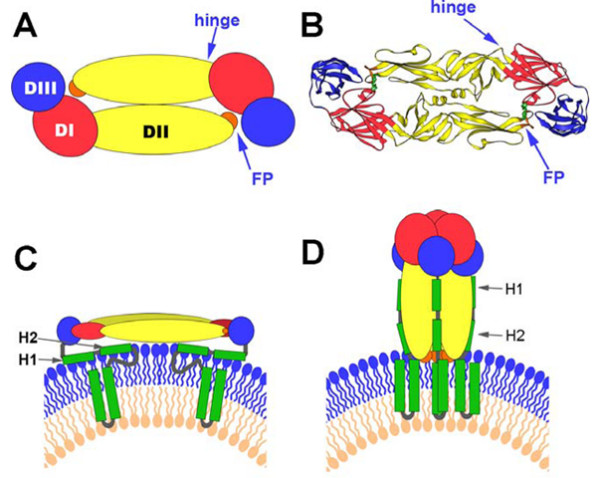
Summary of the Structural Organization and Different Conformations of the Flavivirus Envelope Protein E (obtained the kind permission from the copyright holder to reproduce figures that have previously been published on [51]). (A) Schematic top view of the organization of the sE protein dimer as present at the surface of mature virions, color-coded according to the three domains (DI, DII, and DIII). The fusion peptide (FP) is indicated in orange. (B) Crystal structure (top view) of the TBEV E ectodomain (termed "sE") dimer. (C) Schematic side view of the DV E dimer at the surface of mature virions, with the "stem" and TM C-terminal polypeptide segments (missing in the truncated sE form) indicated in green. The viral lipid bilayer is illustrated with lipids belonging to the outer and inner leaflets colored blue and pink, respectively. Cryo-electron microscopy 3D reconstructions have shown that the stem forms two α-helices (H1 and H2) lying on the viral membrane, followed by the two transmembrane (TM) segments. (D) Schematic representation illustrating the proposed organization of full-length DV E in its postfusion conformation. In this model, the α-helices of the stem interact with the body of the trimer, in the grooves between adjacent, parallel DIIs. The lipid bilayer as well as the stem and TM segments is drawn as in (C).
