Abstract
The genetic diversity of HIV-1 envelope glycoproteins (Env) remains a major obstacle to the development of an antibody-based AIDS vaccine. The present studies examine the breadth and magnitude of neutralizing antibody (NAb) responses in rhesus monkeys after immunization with DNA prime-recombinant adenovirus (rAd) boost vaccines encoding either single or multiple genetically distant Env immunogens, and subsequently challenged with a pathogenic simian-human immunodeficiency virus (SHIV-89.6P). Using a standardized multi-tier panel of reference Env pseudoviruses for NAb assessment, we show that monkeys immunized with a mixture of Env immunogens (clades A, B, and C) exhibited a greater breadth of NAb activity against neutralization sensitive Tier 1 viruses following both vaccination and challenge compared to monkeys immunized with a single Env immunogen (clade B or C). However, all groups of Envvaccinated monkeys demonstrated only limited neutralizing activity against Tier 2 pseudoviruses, which are more characteristic of the neutralization sensitivity of circulating HIV-1. Notably, the development of a post-challenge NAb response against SHIV-89.6P was similar in monkeys receiving either clade B, clade C, or clade A+B+C Env immunogens, suggesting cross-clade priming of NAb responses. In addition, vaccines encoding Env immunogens heterologous to SHIV-89.6P primed for a rapid anamnestic NAb response following infection compared to vaccines lacking an Env component. These results show that DNA/rAd immunization with multiple diverse Env immunogens is a viable approach for enhancing the breadth of NAb responses against HIV-1, and suggest that Env immunogens can prime for anamnestic NAb responses against a heterologous challenge virus.
Keywords: HIV vaccine, envelope glycoprotein, neutralizing antibody, rhesus monkey, SHIV-89.6P
Introduction
An effective vaccine against HIV-1 will likely need to elicit both cellular and humoral immunity to a wide diversity of viral variants. The HIV-1 envelope glycoprotein (Env) is a vaccine immunogen of particular interest because it can serve as a potent target antigen for both T-lymphocyte and antibody responses (Letvin et al., 2004; Mascola et al., 2005b). Inclusion of Env immunogens in an HIV-1 vaccine may help protect against infection by eliciting antibodies capable of neutralizing infectious virus at the time of exposure, or by priming for the rapid development of a secondary neutralizing antibody (NAb) response that may act to limit viral spread within the host (Mascola, 2003; Mc Cann, Song, and Ruprecht, 2005). However, due to the extraordinary genetic diversity of HIV-1 Env, the ability to elicit broadly cross-reactive antibodies that can neutralize a broad range of HIV-1 variants remains a major impediment in AIDS vaccine development (Burton et al., 2004; Nara and Lin, 2005).
Candidate vaccines that utilize Env immunogens derived from a single HIV-1 strain will likely generate an antibody response of limited breadth (Beddows et al., 1999; Belshe et al., 1998; Bures et al., 2000; Mascola et al., 1996). While many strategies are currently being pursued to design novel Env or peptide immunogens that more effectively elicit a broadly cross-reactive NAb response (Coeffier et al., 2000; Gao et al., 2005; Liang et al., 1999; Liao et al., 2006; Pantophlet and Burton, 2006; Scala et al., 1999), to date these efforts have met with only limited success. An alternative approach to increasing the breadth of Env-specific immunity is the use of polyvalent vaccines that contain multiple genetically distinct HIV-1 Env immunogens (Cho et al., 2001; Hurwitz et al., 2005; Wang et al., 2006; Zhan et al., 2005).
We recently investigated the magnitude and breadth of Env-specific cellular and humoral immunity in rhesus macaques immunized with either single or multiple Env immunogens. Monkeys were immunized with DNA prime/recombinant adenovirus (rAd) boost vaccines encoding an SIV Gag-Pol-Nef polyprotein in combination with either a single HIV-1 clade B Env, a single clade C Env, or a mixture of clade A, clade B, and clade C Envs. The immunogenicity of these vaccine regimens and their protective efficacy against a SHIV-89.6P challenge have been described (Seaman et al., 2005). While these studies demonstrated that multiclade Env immunization can elicit a greater breadth of Env-specific antibody binding activity compared to single clade Env immunization, the breadth and potency of NAb activity was less clear. Plasma samples obtained following vaccination were tested against panels of heterologous HIV-1 clade A, B, and C viruses using a single-round-infection flow cytometric assay, and only modest levels of neutralization against some HIV-1 isolates were observed in monkeys immunized with either single or multiple Env immunogens. However, because the majority of viral isolates used in these assays were not well characterized as to their relative sensitivity or resistance to antibody-mediated neutralization, the ability to compare the breadth and potency of NAbs elicited by these vaccine regimens was limited. Furthermore, the capacity of single or multiple heterologous Env immunogens to prime for a secondary NAb response following SHIV-89.6P challenge was not examined. The present studies were initiated to further investigate the breadth and potency of NAb responses that develop in single- or multi-clade Env immunized monkeys following vaccination and SHIV-89.6P challenge. To this end, we employed a multi-tiered approach for standardized NAb assessments that utilized panels of HIV-1 Envpseudotyped viruses representing vaccine-strain and neutralization sensitive viruses (Tier 1), and primary isolate viruses from multiple genetic subtypes (Tier 2) (Mascola et al., 2005a). Furthermore, we have investigated the ability of Env immunogens either genetically matched or mismatched to the SHIV-89.6P virus to prime for an anamnestic NAb response following infection.
Results
Tier 1 virus neutralization
We recently reported the results of a SHIV-89.6P challenge study in rhesus monkeys that evaluated Env specific T-lymphocyte and antibody responses generated by vaccines containing either a single or multiple genetically distinct HIV-1 Env immunogens (Seaman et al., 2005). Five groups of six monkeys each were immunized with plasmid DNA at 0, 4, and 8 weeks followed by a single rAd boost at week 26. Four groups were vaccinated with SIV Gag-Pol-Nef plus either (i) clade B Env (high clade B Env), (ii) one-third dose clade B Env (low clade B Env), (iii) clade C Env (high clade C Env), or (iv) a combination of one-third each clade A, clade B, and clade C Env (clade A+B+C Env). The fifth group of monkeys was immunized with sham plasmid DNA and rAd vectors (control). All monkeys received an intravenous challenge with 50 MID50 of SHIV-89.6P on week 42, 16 weeks following rAd boost immunization.
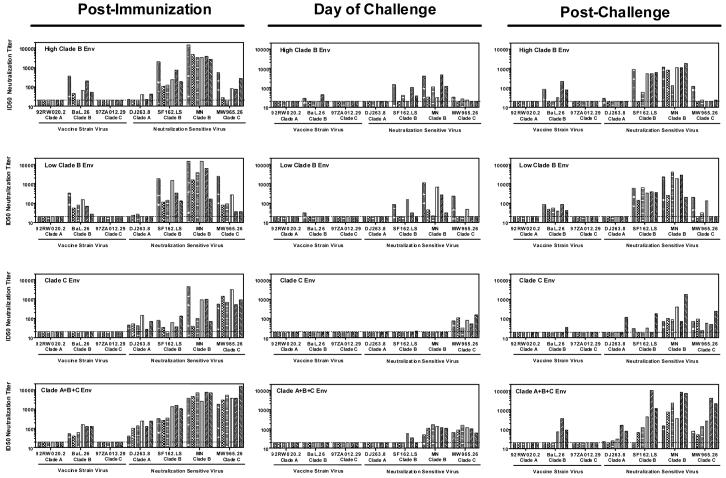
In this study, we first sought to compare the magnitude and breadth of the NAb response generated in these Env-vaccinated monkeys following both immunization and challenge by utilizing a panel of vaccine-strain and neutralization sensitive HIV-1 Env-pseudotyped viruses (Tier 1 viruses). Three of the viruses expressed Envs matching the clade A, B, and C Env immunogens used for vaccination (viruses 92RW020.2, BaL.26, and 97ZA012.29, respectively). Four additional Tier 1 viruses expressed heterologous clade A (DJ263.8), clade B (SF162.LS and MN), or clade C (MW965.26) Envs. These latter viruses are known to be particularly sensitive to antibody-mediated neutralization (J. R. Mascola, unpublished data and (Li et al., 2005). Serial plasma dilutions from individual monkeys were tested against all viruses using a luciferase-based reporter gene assay, and the plasma dilution that produced 50% virus neutralization was determined.
Following DNA prime/rAd boost immunization, monkeys receiving the high- and low-dose clade B Env immunogen demonstrated predominant neutralizing activity against clade B viruses (Figure 1, left panels). While some animals demonstrated comparable cross-neutralization of the clade C virus MW965.26, only weak or undetectable cross-neutralization of the clade A virus DJ263.8 was observed. Plasma samples from all six monkeys immunized with clade C Env vaccine vectors exhibited potent neutralizing activity against the neutralization sensitive clade C virus MW965.26, whereas ID50 titers against the neutralization sensitive clade A and clade B viruses were more variable and generally of lower magnitude. The clade C Env immunized monkeys demonstrated no detectable neutralizing activity against the homologous clade C pseudovirus 97ZA012.29, which we know from previous studies is not easily neutralized. All six monkeys immunized with multiclade Env immunogens demonstrated robust neutralizing activity against all four neutralization sensitive clade A, B, and C viruses. However, of the three vaccine-strain viruses, only the clade B virus BaL.26 was neutralized by plasma from multiclade Env immunized monkeys. Plasma samples from sham vaccinated monkeys had no detectable neutralizing activity against viruses in this Tier 1 panel (data not shown).
Figure 1.
Tier 1 virus neutralization. Plasma samples were obtained from vaccinated monkeys two weeks following rAd boost (Post-Immunization), 16 weeks following rAd boost (Day of Challenge), or six weeks following SHIV-89.6P challenge (Post-Challenge). Serial dilutions of samples were tested for NAb activity against a panel of Tier 1 clade A, B, and C HIV-1 pseudoviruses and TCLA virus (MN). These Tier 1 viruses either express Envs matching the vaccine immunogens, or are known to be sensitive to antibody-mediated neutralization. Data are presented as the ID50 neutralization titer for each of the six monkeys per immunization group. The dashed line represents the assay limit of detection (ID50 titer > 20).
NAb responses during the early stages of infection may prove effective at limiting acute phase viremia. We therefore investigated the magnitude and breadth of the early NAb response in single- or multi-clade Env vaccinated monkeys following SHIV-89.6P challenge. Serum from all groups of Env vaccinated monkeys retained the ability to neutralize Tier 1 viruses on the day of challenge (16 weeks following the rAd boost immunization), although ID50 titers were substantially diminished compared to those measured following immunization (Figure 1, middle panels). Six weeks following SHIV-89.6P infection, monkeys immunized with single clade Env immunogens (either clade B or clade C) demonstrated a secondary NAb response that predominantly neutralized heterologous clade B viruses (Figure 1, right panels). While neutralizing activity against non-clade B viruses was observed in some animals following infection, the ID50 titers were similar to those measured on the day of challenge suggesting that these were not recall NAb responses elicited by the challenge virus. In contrast, 5 of 6 multiclade Env immunized monkeys demonstrated secondary NAb responses following infection that were capable of neutralizing all four heterologous neutralization sensitive clade A, B, and C viruses. Three of six monkeys in the sham vaccinated control group had a detectable NAb response solely against HIV-1 MN at this early post-infection time point (ID50 titers of 90, 149, and 692; data not shown). Together, these data demonstrate that a greater breadth of virus neutralization is detected in multiclade Env vaccinated monkeys compared to single clade Env vaccinated monkeys following both vaccination and challenge when neutralization sensitive Tier 1 viruses are employed.
Tier 2 virus neutralization
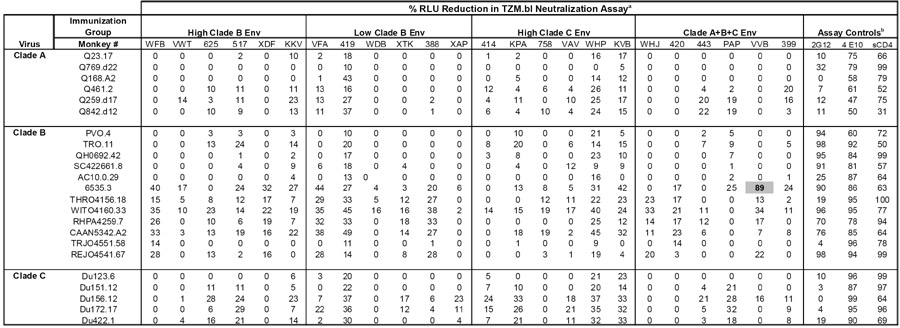
We further evaluated the potency and breadth of the early NAb responses generated in single- or multi-clade Env-vaccinated monkeys following SHIV-89.6P infection by testing sera against additional panels of clade A, B, and C primary isolates. These Tier 2 viruses are not unusually sensitive or resistant to antibody mediated neutralization, and thus allow for a more rigorous assessment of antibody responses elicited following vaccination or infection. Serum samples obtained six weeks following SHIV-89.6P challenge were screened for neutralizing activity against all Tier 2 viruses at a 1:20 dilution. As a baseline control, immune serum from each monkey was assayed in parallel with a corresponding pre-immune sample. Little or no specific neutralizing activity against primary isolate clade A, B, or C pseudoviruses was detected in any of the Env-vaccinated monkeys (Table I) or sham vaccinated monkeys (data not shown). Less than 50% neutralization was observed in all tests, with the exception of serum from monkey VVB (clade A+B+C Env group) which demonstrated 89% neutralization against the clade B virus 6535.3. Further testing of samples obtained at the peak of immunity following DNA prime/rAd boost immunization or at later time points following infection (weeks 8 and 24) also failed to demonstrate any substantial neutralizing activity against these Tier 2 viruses (data not shown). All viruses in these panels demonstrated sensitivity to neutralization by mAbs 2G12 and/or 4E10, and soluble CD4 (Table 1). These data suggest that the NAb responses elicited by either the single clade or multi-clade Env vaccines have limited potency against primary isolates of HIV-1.
Table 1.
Post-challenge neutralization of Tier 2 HIV-1 pseudoviruses.
Samples obtained pre-immunization and six weeks following SHIV-89.6P challenge were screened at a 1:20 dilution in triplicate wells for neutralizing antibody activity against panels of clade A, B, or C Tier 2 HIV-1 pseudoviruses. Data are presented as the percent RLU reduction by post-challenge immune samples in comparison to the corresponding pre-immunization samples. Values >50% RLU reduction are shaded.
Broadly neutralizing monoclonal antibodies 2G12 and 4E10 (50 μg/ml) and soluble CD4 (25 μg/ml) were used as positive control reagents for virus neutralization.
NAb responses against SHIV-89.6P
The Env immunogens used for vaccination in this study are genetically disparate from the 89.6P Env (clade B) expressed by the challenge virus. We therefore examined whether prior immunization with a heterologous Env immunogen from the same clade (high- and low-dose clade B Env groups), a different clade (high clade C group), or multiple clades (clade A+B+C group) impacted the binding Ab and NAb response following SHIV-89.6P challenge. As similar antibody responses were observed in monkeys immunized with either the high- or low-dose clade B Env, data from these animals were combined into one group for these analyses. All groups of Env-vaccinated monkeys had comparable endpoint ELISA titers to HIV-1 gp120 on the day of challenge (Figure 2). Following infection, monkeys in the clade B Env and clade A+B+C Env groups demonstrated a rapid increase in Env-specific antibody titers which peaked approximately four weeks later. In contrast, the anti-gp120 antibody response in clade C Env immunized monkeys appeared to increase with slower kinetics (p< 0.05 for endpoint titer comparisons with clade-B Env or clade A+B+C Env groups at 2 and 4 weeks post-infection, Kruskal-Wallis test). However, this may reflect that a clade B Env antigen (MN) was being used to measure antibody binding activity in these assays. Importantly, all groups of Env-vaccinated monkeys demonstrated comparable end-point ELISA titers eight weeks following SHIV-89.6P challenge. Antibody responses against MN gp120 were detected in 3 of 6 monkeys in the control group beginning on week 4 post-challenge.
Figure 2.
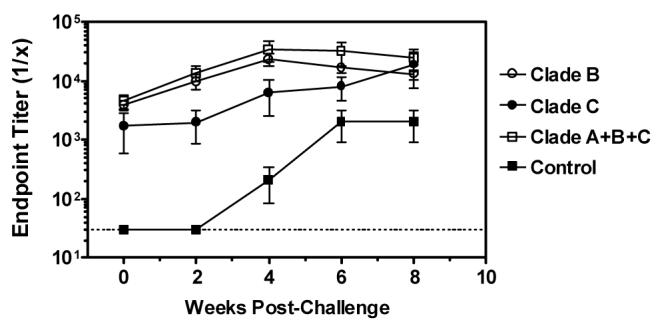
Post-challenge anti-Env antibody binding titers. Serum samples were obtained from vaccinated and control monkeys on the day of SHIV-89.6P challenge and subsequent time points following infection. Anti-gp120 antibody binding titers were determined by ELISA. Data are presented as the mean endpoint titers +/− SEM. The dashed line represents the assay limit of detection (endpoint titer >30).
None of the Env-vaccinated animals had detectable serum neutralizing activity against SHIV-89.6P prior to challenge. The emergence of a NAb response against the challenge virus was therefore monitored in all groups of monkeys following infection (Figure 3). At four weeks post-challenge, 16 of 24 monkeys previously immunized with Env-encoding vaccines had detectable ID50 NAb titers against SHIV-89.6P, and all 24 animals had developed a detectable response within six weeks of infection. At week 6 post-infection, titers of NAbs against SHIV-89.6P in Env-vaccinated monkeys were significantly higher than those observed in sham vaccinated monkeys (p = 0.02, Kruskal-Wallis test). No significant differences were seen between the groups of monkeys immunized with single clade or multiclade Env immunogens in terms of either the kinetics of the response or the magnitude of ID50 neutralization titers (p >0.05, Kruskal-Wallis test).
Figure 3.
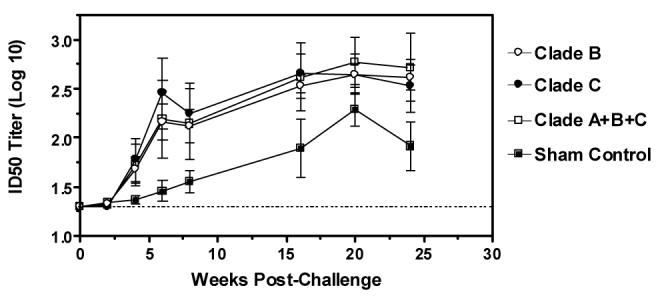
Post-challenge NAb responses against SHIV-89.6P. Serum samples were obtained from vaccinated and control monkeys on the day of SHIV-89.6P challenge and subsequent time points following infection. Serial dilutions of samples were tested for NAb activity against SHIV-89.6P. Data are presented as the mean ID50 neutralization titer +/− SEM. The dashed line represents the assay limit of detection (ID50 titer > 20).
In the absence of a detectable NAb response against SHIV-89.6P following immunization, it is difficult to ascertain whether the anti-SHIV-89.6P response that developed in these monkeys following challenge can be attributed to a secondary serum antibody response. As these vaccines have previously been shown to blunt the loss of CD4+ T-lymphocytes following challenge (Seaman et al., 2005), it remains possible that the anti-SHIV-89.6P NAb response that develops in vaccinated monkeys reflects a primary response that is generated in the setting of preserved CD4+ T cell help.
To further investigate this issue, we performed a retrospective comparison study utilizing cohorts of rhesus monkeys from four separate vaccine studies that utilized a SHIV-89.6P challenge (further detailed in Materials and Methods). In order to maximize the power to detect significant differences in the development of a true anamnestic NAb response against the challenge virus, monkeys from these studies were grouped according to the Env component of the vaccine they received. Vaccinated monkeys were divided into groups that had been immunized with vectors expressing either SIV Gag/Pol plus a genetically matched 89.6P Env (Match Env, n=52), SIV Gag/Pol plus a genetically mismatched Env(s) (Mismatch Env, n=30), or SIV Gag/Pol alone with no Env component (Mock Env, n=6). Sham vaccinated monkeys from all four studies were compiled as a control group (n=46). All monkeys received an intravenous challenge with 50 MID50 SHIV-89.6P between weeks 38 and 60 post-immunization. The same stock of challenge virus was used in all four studies. None of the vaccinated or control monkeys had detectable serum NAb activity against SHIV-89.6P following vaccination or prior to challenge (data not shown). Analysis of serum samples obtained two weeks following challenge demonstrated a rapid anamnestic NAb response against SHIV-89.6P in the Match Env group, with 32 of the 52 monkeys having detectable ID50 titers ranging from 24 to 181 (Figure 4). By week 4 post-challenge, the ID50 neutralization titers remained significantly higher in the Match Env group (median titer of 220) when compared to responses measured in the Mismatch Env group (median titer of 29), the Mock Env group (median titer of 20), or the Control group (median titer of 20). Further analysis of the response rate four weeks following infection demonstrates that 17 of 30 monkeys in the Mismatch Env group had detectable neutralizing activity against SHIV-89.6P compared to only 1 of 6 monkeys in the Mock Env group and 4 of 46 monkeys in the Control group (Table 2). While the statistical power to detect significant differences between the Mismatch and Mock Env groups was limited due to the small number of monkeys in the Mock Env group (p = 0.089, Fisher's exact test), these data are suggestive that inclusion of an Env immunogen genetically disparate from the challenge virus may have contributed to the development of a more rapid NAb response following infection. By weeks 6 and 8 post-challenge, no significant differences in the response rates or median neutralization titers were detected between monkeys in the Match, Mismatch, or Mock Env groups. The development of a NAb response to SHIV-89.6P was also observed in a subset of monkeys in the control group at these later time points. It should be noted that although these vaccination groups were comprised of monkeys from four separate vaccine studies, the number of animals that did or did not develop a response following infection was not biased to a particular study. Together, these data suggest that vaccines containing an Env immunogen can prime for a rapid anamnestic NAb response following infection compared to similar vaccines lacking an Env component.
Figure 4.
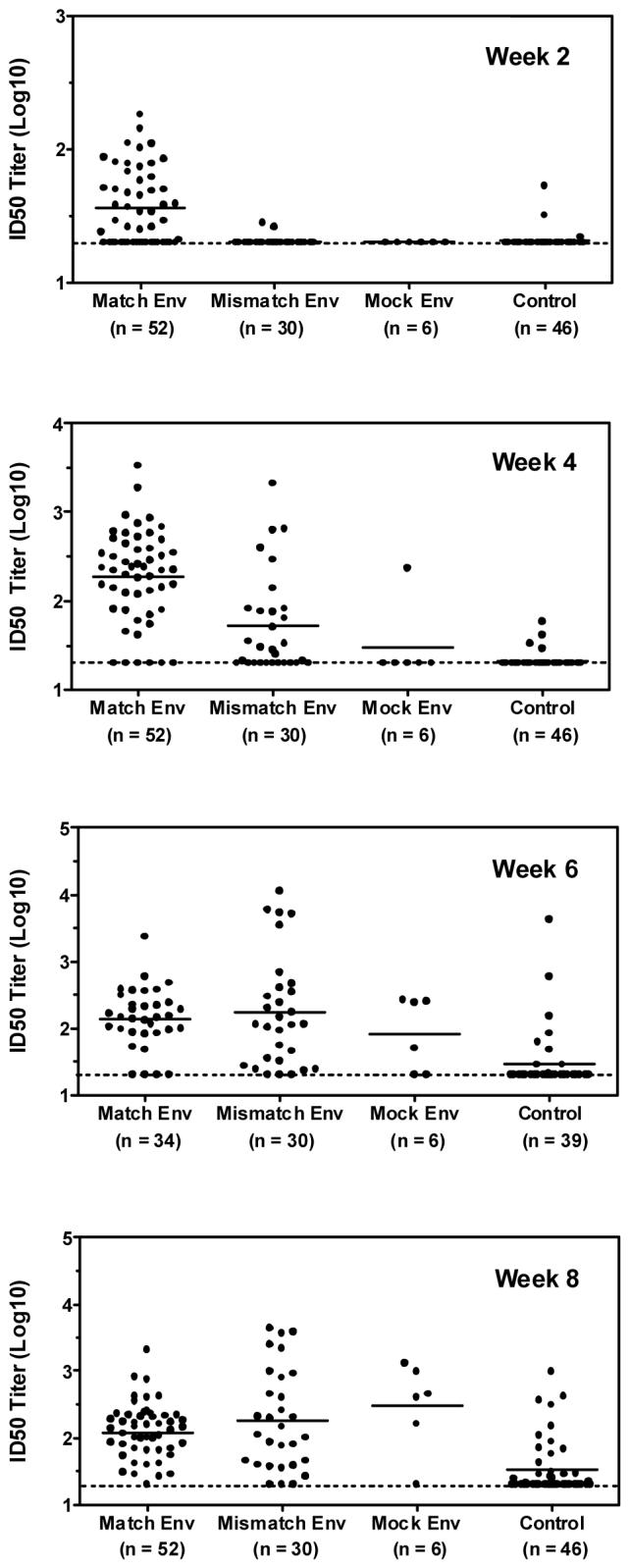
Post-challenge kinetics of the anti-SHIV-89.6P NAb response in Env vaccinated and non-Env vaccinated monkeys. Anti-SHIV-89.6P NAb titers were compared in groups of vaccinated monkeys immunized with vectors expressing Gag/Pol and 89.6P Env (Match Env), Gag/Pol and heterologous Env(s) (Mismatch Env), Gag/Pol alone (Mock Env), or sham vaccine vectors (Control). Data are presented as the serum ID50 neutralization titer for individual monkeys, with bars indicating the geometric mean titer for each group. Note that the number of monkeys available for analysis in the Match Env and Control groups is lower at the week 6 post-challenge time point due to the lack of serum samples available from one of the four vaccination studies used for these analyses.
Table 2.
Response rate comparisons four weeks post-challenge.
| Responders (%)a | p valueb | |
|---|---|---|
| Match (88) | vs Mismatch (56) | 0.0015 |
| vs Mock (17) | 0.0005 | |
| vs Control (2) | <0.0001 | |
| Mismatch (56) | vs Mock (17) | 0.089 |
| vs Control (2) | <0.0001 | |
| Mock (17) | vs Control (2) | 0.472 |
The percentage of monkeys in each group that had an anti-SHIV-89.6P ID50 titer >20 at 4 weeks following challenge was determined, and is indicated in parenthesis.
P values were determined using a Fisher's exact t-test.
Discussion
We have evaluated the breadth and potency of NAb responses in monkeys immunized with DNA prime/rAd boost vaccines encoding single or multiple Env immunogens and subsequently challenged with SHIV-89.6P. In contrast to our previous study (Seaman et al., 2005), we employed a standardized multi-tiered approach for NAb assessment that utilized a panel of viruses known to be sensitive to antibody-mediated neutralization (Tier 1), and an additional panel of well-characterized reference strains that are representative of circulating virus strains (Tier 2). In this manner, antibody-based vaccine immunogens can be evaluated for the ability to elicit broadly cross-reactive NAbs, and standardized data sets can be generated to identify promising candidates for further development (Mascola et al., 2005a). Our data suggest that a combination of multiple genetically disparate Env immunogens (clades A, B, and C) can elicit a greater breadth of NAb activity against Tier 1 viruses following both vaccination and challenge compared to a single clade Env immunogen (either clade B or C). While single clade Env vaccinated monkeys demonstrated potent neutralizing activity against heterologous Tier 1 viruses matched in clade to the Env immunogen they received, the magnitude and specificity of cross-clade virus neutralization varied greatly between individual animals. In contrast, all six monkeys immunized with multiclade Env immunogens had equivalent, robust neutralization titers against heterologous clade A, B, and C Tier 1 viruses following immunization. Importantly, these monkeys further demonstrated a greater breadth of Tier 1 virus neutralization following a pathogenic challenge. Robust secondary NAb responses were observed in multiclade Env vaccinated animals following SHIV-89.6P infection that were capable of cross-neutralizing DJ263.8 (clade A) and MW965.26 (clade C) viruses in addition to the heterologous clade B viruses SF162.LS and MN. In contrast, post-challenge secondary NAb responses elicited in monkeys immunized with single clade Env immunogens (either clade B or C) predominantly neutralized only the clade B viruses. Additional studies to examine the epitope specificities of neutralizing antibodies elicited by these vaccines may enhance our understanding of the mechanisms underlying the breadth of these responses. Whether polyvalent Env immunization generates antibodies to a greater number of antigenically variable neutralization epitopes or increases the cross-reactive potential of antibodies directed against more conserved epitopes remain to be determined.
Because this study did not include a group of monkeys immunized with the clade A Env immunogen alone, we cannot exclude the possibility that the enhanced breadth observed in the multiclade Env group is attributed to the inclusion of the clade A Env immunogen. However, we have previously analyzed NAb responses elicited in guinea pigs immunized with the clade A Env immunogen alone and found NAb activity against some clade A and clade C Tier 1 viruses, but little cross-neutralization against clade B Tier 1 viruses (J. Mascola, unpublished data). We therefore believe it unlikely that the enhanced breadth of NAb responses observed in the multiclade Env group can solely be attributed to the inclusion of the clade A Env immunogen.
Sera from monkeys immunized with the clade A or clade C Env immunogens were not able to neutralize the vaccine strain viruses. It is possible that these viruses are particularly resistant to neutralization or that vaccination did not elicit antibodies with the specificities required for neutralization. Nonetheless, these results illustrate the utility of including heterologous neutralization sensitive viruses in Tier 1 virus panels, and suggest that initial immunological assessment of novel Env immunogens should not be solely based on the ability to elicit antibodies which can neutralize homologous viruses.
Tier 1 virus neutralization should be viewed as a minimum requirement for antibody-based vaccine immunogens (Mascola et al., 2005a). A greater measure of the potency and breadth of NAb responses can be assessed using panels of Tier 2 HIV-1 pseudoviruses. We were unable to detect any significant neutralization against clade A, B, or C viruses following either vaccination (data not shown) or acute infection (Table 1) in monkeys immunized with single or multiple Env immunogens. The clade B viruses used for these analyses represent an initial panel of reference strains that were recently described (Li et al., 2005). These viruses are not considered unusually neutralization-resistant, as each is sensitive to neutralization by sCD4, one or more broadly reactive mAbs, and a subset of serum samples from HIV-1 infected individuals. While a standard reference panel of HIV-1 clade A Env clones has yet to be developed, a standardized panel of clade C Env clones has recently been described (Li et al., 2006). Three of the five clade C Tier 2 viruses used in the present study are included in that panel (viruses Du156.12, Du172.17, and Du422.1). All of the Tier 2 clade A and clade C viruses used in our panels also demonstrate moderate sensitivity to neutralization by broadly reactive mAbs and sCD4, and thus most likely do not underestimate the potency of neutralizing antibodies elicited following vaccination or challenge. Whether continued efforts to develop novel immunogens that present more conserved regions of the Env protein will prove successful at enhancing the potency and breadth of vaccine-elicited neutralizing antibodies remains to be seen (Pantophlet and Burton, 2006). Optimizing the methods of vaccine delivery may also warrant further examination. Recent reports have suggested that inclusion of a recombinant protein boost in a vaccine regimen can effectively increase the breadth and potency of NAb responses directed against HIV-1 (Shu et al., 2007; Wang et al., 2006).
The ability of a vaccine to prime for a rapid secondary antibody response during the early stages of infection may prove beneficial in limiting the initial spread of virus. This study further provided an opportunity to evaluate the anamnestic NAb response that developed in single- or multiclade-Env immunized monkeys following a pathogenic SHIV challenge. While we could not detect the presence of serum NAbs against SHIV-89.6P following immunization, all Env-vaccinated monkeys demonstrated a rapid and robust NAb response to the challenge virus following infection. Importantly, the magnitude and kinetics of these responses were equivalent in monkeys previously immunized with either a single Env immunogen (either matched or mismatched in clade to the challenge virus), or multiple Env immunogens.
It may be argued that the NAb response that develops in vaccinated monkeys following SHIV-89.6P infection reflects a de novo response that is generated in the setting of preserved or primed CD4+ T lymphocyte help rather than a true secondary response that was primed by vaccination. While the presence of T-cell help may certainly potentiate the development of a NAb response following SHIV-89.6P infection, we demonstrate here that prior immunization with an Env immunogen can indeed prime for a rapid recall response. Data compiled from four similar HIV-1 vaccine studies in rhesus monkeys demonstrated that over 60% of animals immunized with vectors expressing Gag/Pol and 89.6P Env (Match Env group) developed a detectable NAb response within two weeks following challenge, while the majority of monkeys immunized with Gag/Pol alone (Mock Env group) did not develop a response until approximately six weeks following challenge. Importantly, we also observed a trend suggesting that monkeys immunized with heterologous Env immunogens (Mismatch Env group) developed a more rapid NAb response to the challenge virus when compared to monkeys in the Mock Env group. As all groups of vaccinated monkeys in these studies demonstrated comparable levels of protection against viral replication and CD4+ T lymphocyte loss (Letvin et al., 2004; Santra et al., 2004; Seaman et al., 2005), these data support the notion that rapid NAb responses in the Match Env group of monkeys, and perhaps the Mismatch Env group, can be attributed to the presence of vaccine-primed B cells. These data further support prior observations that active or passive immunization of rhesus monkeys can accelerate the development of neutralizing antibody responses following SHIV-SF162P4 or SIVsmE660 challenge. (Buckner et al., 2004; Haigwood et al., 2004; Ourmanov et al., 2000).
The protection afforded by vaccine-elicited antibodies in the SHIV-89.6P challenge model is more difficult to ascertain. Despite the rapid development of a NAb response in the Match Env group of monkeys, we could not detect a significant correlation between NAb titers and plasma viral RNA levels or CD4+ T lymphocyte counts in individual animals. Furthermore, no significant differences in these parameters of infection were detected between the Match, Mismatch, or Mock Env groups of vaccinated monkeys (data not shown). As previously reported, the vaccines utilized in these studies prime for a robust anamnestic cellular immune response following SHIV-89.6P infection. As protection against viral replication and CD4+ T-lymphocyte loss most likely reflect the contributions of both virus-specific T-lymphocyte and antibody mediated immunity, it is difficult to specifically define the protective contribution of vaccine-elicited binding antibodies or NAbs in this challenge model.
The results presented here demonstrate that a polyvalent-Env AIDS vaccine is a viable approach for increasing the breadth of antibody mediated immunity. However, the data also highlight the limitations of current Env-based immunogens to elicit antibodies with the potency to neutralize a diversity of HIV-1 primary isolates. We have further shown the utility of a standardized multi-tiered approach for NAb assessment. As novel antibody-based vaccine immunogens are developed, such an approach will prove important for detecting incremental advances in the potency and breadth of vaccine-elicited NAb responses against HIV-1.
Materials and Methods
Immunization and challenge of rhesus monkeys
Thirty adult Indian-origin rhesus monkeys (Macaca mulatta) were divided into five groups of six animals each for DNA prime/recombinant replication-defective adenovirus boost immunizations (Seaman et al., 2005). Plasmid DNA and rAd vaccine vectors were constructed as previously described (Kong et al., 2003; Letvin et al., 2004). Plasmid DNA vaccines were administered by intramuscular injection using a needle-free Biojector system and a no. 3 syringe (Bioject, Portland, OR) at 0, 4, and 8 weeks. For each DNA immunization, monkeys received 4.5 mg of SIVmac239 gag/pol/nef plasmid and 4.5 mg of HIV-1 env plasmid (s) (9 mg total). For the HIV-1 Env component of the vaccine, four groups of monkeys received either 4.5 mg of HXBc2/BaL clade B env (high clade B Env group), 1.5 mg of HXBc2/BaL clade B env plus 3.0 mg of sham plasmid (low clade B Env group), 4.5 mg of clade C env (high clade C Env group), or a mixture of 1.5 mg each of clade A, B, and C env plasmids (clade A+B+C Env group). Plasmid DNAs were divided into two aliquots of 0.5 ml each and delivered into each quadriceps muscle. At week 26, monkeys received a boost immunization with rAd vectors expressing immunogens matching those used for the DNA prime. A total of 2 × 1012 rAd particles (1 × 1012 rAd-SIVmac239 gag/pol and 1 × 1012 rAd-HIV-1 env[s]) were delivered by intramuscular injection as described above. One group of control monkeys were immunized with sham DNA at weeks 0, 4, and 8 and boosted with sham rAd vectors at week 26. At week 42, all monkeys received an intravenous challenge with 50- 50% monkey infectious doses (MID50) of SHIV-89.6P.
Kinetic analyses of the post-challenge NAb response against SHIV-89.6P were performed using previously described cohorts of monkeys that were immunized with vectors expressing SIVmac239 gag or gag/pol together with either an Env component matching the challenge virus (Match Env group), an Env(s) component heterologous to the challenge virus (Mismatch Env group), or a sham vector lacking an Env component (Mock Env group). The 52 monkeys in the Match Env group were compiled from 3 independent vaccine studies. Twenty-eight monkeys (7 monkeys per group) were immunized with SIVmac239 gag and HIV-1 89.6P env as a DNA prime/DNA boost, a DNA prime/recombinant Vaccinia virus (rVac) boost, a DNA prime/recombinant Modified Vaccinia Ankara (rMVA) boost, or a DNA prime/recombinant fowlpox (rFPV) boost (Santra et al., 2004). Eighteen monkeys (6 monkeys per group) were immunized with SIVmac239 gag and HIV-1 89.6P env as an rVac prime/rFPV boost, an rMVA prime/rFPV boost, or an rMVA prime/rMVA boost (S. Santra and N. L. Letvin, unpublished data). The remaining 6 monkeys in the Match Env group were immunized with SIVmac239 gag/pol and HIV-1 89.6P env as a DNA prime/rAd boost (Letvin et al., 2004). The 30 monkeys in the Mismatch Env group were compiled from 2 independent vaccine studies. Twenty-four of the monkeys were from the multiclade Env vaccine study described above. The remaining 6 monkeys were immunized with SIVmac239 gag/pol and HxBc2/BaL env as a DNA prime/rAd boost (Letvin et al., 2004). The 6 monkeys in the Mock Env group were also from this latter study, and received DNA prime/rAd boost immunizations with SIVmac 239 gag/pol vectors alone. The 46 monkeys in the Control group were sham vector immunized animals compiled from all 4 studies previously mentioned. All vaccinated and control monkeys received an intravenous challenge with 50 MID50 of SHIV-89.6P. The same challenge stock of SHIV-89.6P virus was used in all 4 vaccine studies.
Cell lines
The TZM.bl cell line was obtained through the NIH AIDS Research and Reference Reagent Program (ARRRP), as contributed by Drs. J. Kappes and X. Wu (Derdeyn et al., 2000; Platt et al., 1998). 293T/17 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Both of these adherent cell lines were maintained in D-MEM growth medium (Gibco/Invitrogen) containing 10% heat-inactivated fetal bovine serum (FBS), 25 mM HEPES, and 50 μg/ml gentamicin. Cells were harvested using Trypsin/EDTA solution (Gibco/Invitrogen). H9 cells were cultured in RPMI-1640 medium (Cellgro, Herndon, VA) containing 20% heat-inactivated FBS, 25 mM HEPES, and 50ug/ml gentamicin. All cell lines were maintained at 37° C in humidified air containing 5% CO2.
HIV-1 Env-pseudotyped viruses and replication competent viral stocks
Stocks of single-round infection HIV-1 Env-pseudovirus were produced by co-transfecting 293T/17 cells (5 × 106 cells per T75 flask) with 10 μg of a HIV-1 rev/env expression plasmid and 10 μg of an env-deficient HIV-1 backbone plasmid (pSG3ΔEnv) using CellPhect transfection reagent (Amersham Biosciences, Buckinghamshire, England). Pseudovirus-containing supernatant was harvested 48 hours following transfection and clarified by centrifugation and 0.45-micron filtration. Single use aliquots (1.0 ml) were stored at −80° C. The 50% tissue culture infectious dose (TCID50) for each pseudovirus preparation was determined by infection of TZM.bl cells as previously described (Li et al., 2005).
Stocks of HIV-1 pseudovirus used for Tier 1 virus neutralization assessment were generated using HIV-1 env/rev expression plasmids obtained as follows: plasmids expressing an HIV-1 Env matching the vaccine immunogens for clade A (92RW020.2), clade B (BaL.26), and clade C (97ZA012.29), as well as the plasmid expressing a neutralization sensitive heterologous Env for clade A (DJ263.8) were provided by J. Mascola (NIH/Vaccine Research Center). A plasmid expressing the heterologous neutralization sensitive clade C Env MW965.26 was obtained from the NIH ARRRP, as provided by Dr. B. Hahn (Gao et al., 1996). A plasmid expressing the heterologous neutralization sensitive clade B Env SF162.LS (Stamatatos, Wiskerchen, and Cheng-Mayer, 1998) was provided by L. Stamatatos (Seattle Biomedical Research Institute). For Tier 2 virus neutralization assessment, the 6 subtype A HIV-1 Env clones Q842ENVd12, Q461ENVe2, Q168ENVa2, Q769ENVd22, Q23ENV17, and Q259ENVd2.17 were obtained through the NIH ARRRP, as contributed by Dr. J. Overbaugh (Long et al., 2002; Poss and Overbaugh, 1999). The following standard reference panel of 12 subtype B HIV-1 Env clones (Li et al., 2005) was also obtained through the NIH ARRRP: 6535.3, QH0692.42, PVO.4, TRO.11, AC10.0.29, SC422661.8 (contributed by Drs. D. Montefiori, F. Gao, and M. Li); WITO4160.33, REJO4541.67, RHPA4259.7 (contributed by Drs. B. Hahn and J. Salazar-Gonzalez); TRJO4551.58 (contributed by Drs. B. Hahn, X. Wei, and G. Shaw); and THRO4156.18, CAAN5342.A2 (contributed by Drs. B. Hahn and D. Kothe). The 5 subtype C HIV-1 Env clones Du123.6, Du151.2, Du156.12, Du172.17, and Du422.1 have been described (Li et al., 2006).
A T cell line adapted (TCLA) strain of HIV-1MN was obtained from the NIH ARRRP as contributed by R. Gallo (Gallo et al., 1984; Shaw et al., 1984); and cell free stocks were generated using H9 cells as previously described (Montefiori et al., 1988). Stocks of cell free SHIV-89.6P virus used for in vitro neutralization assays were generated using phytohemagglutinin and interleukin-2 stimulated human peripheral blood mononuclear cells as previously described (Crawford et al., 1999).
Serum and plasma samples, soluble CD4, and monoclonal antibody (mAb) reagents
All monkey serum and plasma samples were heat-inactivated at 56° C for 1 hour and clarified by centrifugation prior to use. Recombinant soluble CD4 (sCD4) was purchased from Progenics Pharmaceuticals, Inc. (Tarrytown, NY). The human anti-HIV-1 mAbs 4E10 and 2G12 were obtained from the NIH ARRRP, as contributed by H. Katinger (Stiegler et al., 2001; Trkola et al., 1996).
HIV-1 envelope antibody enzyme-linked immunosorbent assay (ELISA)
Recombinant HIV-1MN envelope glycoprotein gp120 was obtained from ImmunoDiagnostics, Inc. (Woburn, MA). Ninety-six-well Maxisorp ELISA plates (Nunc, Roskilde, Denmark) were coated overnight at 4° C with 100 μl carbonate coating buffer containing 1 μg/ml gp120 antigen. Plates were washed 3 times with PBS/0.05% Tween-20 wash buffer and blocked for 2 hours with a blocking buffer containing 5% nonfat dry milk, 5% FBS, and 0.05% Tween-20 in PBS. Serum samples were serially diluted in blocking buffer and added to the ELISA plate in duplicate wells (100 μl/well). A pre-immune negative control serum sample was tested in parallel with the corresponding post-challenge samples for each monkey. The plates were incubated for 2 hours at RT, washed 5 times, and a secondary peroxidase-labeled goat anti-monkey IgG antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) diluted 1:2,500 in blocking buffer was added (100 μl/well). Plates were incubated for 1 hour at RT, washed 5 times, and developed with TMB substrate (Kirkegaard & Perry) for 15 minutes. The reaction was stopped by the addition of TMB stop solution and the plates were analyzed at 450 nm with a Multiskan EX ELISA reader (Thermo Electron, Vantaa, Finland). The endpoint titer for each monkey was established as the last dilution with a corrected optical density >0.1 after subtraction of pre-immune background values.
Neutralization Assays
NAb responses against SHIV-89.6P and HIV-1 viruses were measured using a luciferase-based assay in TZM.bl cells as previously described (Li et al., 2005; Montefiori, 2004). This assay measures the reduction in luciferase reporter gene expression in TZM.bl cells following a single round of virus infection. Briefly, 3-fold serial dilutions of serum samples were performed in triplicate (96-well flat bottom plate) in 10% D-MEM growth medium (100 μl/well). 200 TCID50 of virus was added to each well in a volume of 50 μl and the plates were incubated for 1 hour at 37° C. TZM.bl cells were then added (1×104/well in 100 μl volume) in 10% D-MEM growth medium containing DEAE-Dextran (Sigma, St. Louis, MO) at a final concentration of 11 μg/ml. For assays utilizing the replication-competent viruses HIV-1MN and SHIV-89.6P, Indinavir was added at a final concentration of 1 μM to prevent secondary rounds of infection. Assay controls included replicate wells of TZM.bl cells alone (cell control) and TZM.bl cells with virus (virus control). Following a 48 hour incubation at 37° C, 150 μl of assay medium was removed from each well and 100 μl of Bright-Glo luciferase reagent (Promega, Madison, WI) was added. The cells were allowed to lyse for 2 minutes, then 150 μl of the cell lysate was transferred to a 96-well black solid plate and luminescence was measured using a Victor 3 luminometer (Perkin Elmer). The 50% inhibitory dose (ID50) titer was calculated as the serum dilution that caused a 50% reduction in relative luminescence units (RLU) compared to the virus control wells after subtraction of cell control RLUs.
For analysis of neutralizing activity against Tier 2 viruses, post-challenge serum samples were screened in triplicate at a 1:20 dilution in parallel with the corresponding pre-immune sample. Data are reported as the percent RLU reduction in wells containing post-challenge serum relative to RLU in wells containing pre-immune plasma.
Statistical Analysis
The nonparametric Kruskal-Wallis test was used for multiple group comparisons of ID50 NAb titers. Differences between groups were further analyzed by Mann-Whitney test. Response rates were compared using Fisher's exact test. All tests were performed using GraphPad Prism software, version 4.0.
Acknowledgments
This work was supported in part by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH and NIH grant AI-30033.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beddows S, Lister S, Cheingsong R, Bruck C, Weber J. Comparison of the antibody repertoire generated in healthy volunteers following immunization with a monomeric recombinant gp120 construct derived from a CCR5/CXCR4-using human immunodeficiency virus type 1 isolate with sera from naturally infected individuals. J Virol. 1999;73(2):1740–5. doi: 10.1128/jvi.73.2.1740-1745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe RB, Gorse GJ, Mulligan MJ, Evans TG, Keefer MC, Excler JL, Duliege AM, Tartaglia J, Cox WI, McNamara J, Hwang KL, Bradney A, Montefiori D, Weinhold KJ. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. Aids. 1998;12(18):2407–15. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- Buckner C, Gines LG, Saunders CJ, Vojtech L, Srivastava I, Gettie A, Bohm R, Blanchard J, Barnett SW, Safrit JT, Stamatatos L. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology. 2004;320(1):167–80. doi: 10.1016/j.virol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Bures R, Gaitan A, Zhu T, Graziosi C, McGrath KM, Tartaglia J, Caudrelier P, El Habib R, Klein M, Lazzarin A, Stablein DM, Deers M, Corey L, Greenberg ML, Schwartz DH, Montefiori DC. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2000;16(18):2019–35. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- Cho MW, Kim YB, Lee MK, Gupta KC, Ross W, Plishka R, Buckler-White A, Igarashi T, Theodore T, Byrum R, Kemp C, Montefiori DC, Martin MA. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous Simian/human immunodeficiency virus infection in pigtailed macaques. J Virol. 2001;75(5):2224–34. doi: 10.1128/JVI.75.5.2224-2234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coeffier E, Clement JM, Cussac V, Khodaei-Boorane N, Jehanno M, Rojas M, Dridi A, Latour M, El Habib R, Barre-Sinoussi F, Hofnung M, Leclerc C. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine. 2000;19(78):684–93. doi: 10.1016/s0264-410x(00)00267-x. [DOI] [PubMed] [Google Scholar]
- Crawford JM, Earl PL, Moss B, Reimann KA, Wyand MS, Manson KH, Bilska M, Zhou JT, Pauza CD, Parren PW, Burton DR, Sodroski JG, Letvin NL, Montefiori DC. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73(12):10199–207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74(18):8358–67. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224(4648):500–3. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gao F, Morrison SG, Robertson DL, Thornton CL, Craig S, Karlsson G, Sodroski J, Morgado M, Galvao-Castro B, von Briesen H, Beddows S, Weber J, Sharp PM, Shaw GM, Hahn BH. Molecular cloning and analysis of functional envelope genes from human immunodeficiency virus type 1 sequence subtypes A through G. The WHO and NIAID Networks for HIV Isolation and Characterization. J Virol. 1996;70(3):1651–67. doi: 10.1128/jvi.70.3.1651-1667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Weaver EA, Lu Z, Li Y, Liao HX, Ma B, Alam SM, Scearce RM, Sutherland LL, Yu JS, Decker JM, Shaw GM, Montefiori DC, Korber BT, Hahn BH, Haynes BF. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J Virol. 2005;79(2):1154–63. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigwood NL, Montefiori DC, Sutton WF, McClure J, Watson AJ, Voss G, Hirsch VM, Richardson BA, Letvin NL, Hu SL, Johnson PR. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J Virol. 2004;78(11):5983–95. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz JL, Slobod KS, Lockey TD, Wang S, Chou TH, Lu S. Application of the polyvalent approach to HIV-1 vaccine development. Curr Drug Targets Infect Disord. 2005;5(2):143–56. doi: 10.2174/1568005054201517. [DOI] [PubMed] [Google Scholar]
- Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77(23):12764–72. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Huang Y, Chakrabarti BK, Xu L, Seaman MS, Beaudry K, Korioth-Schmitz B, Yu F, Rohne D, Martin KL, Miura A, Kong WP, Yang ZY, Gelman RS, Golubeva OG, Montefiori DC, Mascola JR, Nabel GJ. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J Virol. 2004;78(14):7490–7. doi: 10.1128/JVI.78.14.7490-7497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol. 2006;80(23):11776–90. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Munshi S, Shendure J, Mark G, 3rd, Davies ME, Freed DC, Montefiori DC, Shiver JW. Epitope insertion into variable loops of HIV-1 gp120 as a potential means to improve immunogenicity of viral envelope protein. Vaccine. 1999;17(22):2862–72. doi: 10.1016/s0264-410x(99)00125-5. [DOI] [PubMed] [Google Scholar]
- Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma BJ, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353(2):268–82. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EM, Rainwater SM, Lavreys L, Mandaliya K, Overbaugh J. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res Hum Retroviruses. 2002;18(8):567–76. doi: 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- Mascola JR, D'Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol. 2005a;79(16):10103–7. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Sambor A, Beaudry K, Santra S, Welcher B, Louder MK, Vancott TC, Huang Y, Chakrabarti BK, Kong WP, Yang ZY, Xu L, Montefiori DC, Nabel GJ, Letvin NL. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J Virol. 2005b;79(2):771–9. doi: 10.1128/JVI.79.2.771-779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, Clements ML, Dolin R, Graham BS, Gorse GJ, Keefer MC, McElrath MJ, Walker MC, Wagner KF, McNeil JG, McCutchan FE, Burke DS. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173(2):340–8. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. In: Coligan AMKJE, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. John Wiley & Sons; New York: 2004. pp. 12.11.1–12.11.15. [DOI] [PubMed] [Google Scholar]
- Montefiori DC, Robinson WE, Jr., Schuffman SS, Mitchell WM. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26(2):231–5. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourmanov I, Bilska M, Hirsch VM, Montefiori DC. Recombinant modified vaccinia virus ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol. 2000;74(6):2960–5. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–69. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss M, Overbaugh J. Variants from the diverse virus population identified at seroconversion of a clade A human immunodeficiency virus type 1-infected woman have distinct biological properties. J Virol. 1999;73(7):5255–64. doi: 10.1128/jvi.73.7.5255-5264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Barouch DH, Korioth-Schmitz B, Lord CI, Krivulka GR, Yu F, Beddall MH, Gorgone DA, Lifton MA, Miura A, Philippon V, Manson K, Markham PD, Parrish J, Kuroda MJ, Schmitz JE, Gelman RS, Shiver JW, Montefiori DC, Panicali D, Letvin NL. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc Natl Acad Sci U S A. 2004;101(30):11088–93. doi: 10.1073/pnas.0401954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G, Chen X, Liu W, Telles JN, Cohen OJ, Vaccarezza M, Igarashi T, Fauci AS. Selection of HIV-specific immunogenic epitopes by screening random peptide libraries with HIV-1-positive sera. J Immunol. 1999;162(10):6155–61. [PubMed] [Google Scholar]
- Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A, Sambor A, Chakrabarti BK, Huang Y, Bailer R, Koup RA, Mascola JR, Nabel GJ, Letvin NL. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;79(5):2956–63. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Hahn BH, Arya SK, Groopman JE, Gallo RC, Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984;226(4679):1165–71. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Shu Y, Winfrey S, Yang ZY, Xu L, Rao SS, Srivastava I, Barnett SW, Nabel GJ, Mascola JR. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25(8):1398–408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L, Wiskerchen M, Cheng-Mayer C. Effect of major deletions in the V1 and V2 loops of a macrophage-tropic HIV type 1 isolate on viral envelope structure, cell entry, and replication. AIDS Res Hum Retroviruses. 1998;14(13):1129–39. doi: 10.1089/aid.1998.14.1129. [DOI] [PubMed] [Google Scholar]
- Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2001;17(18):1757–65. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70(2):1100–8. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Pal R, Mascola JR, Chou TH, Mboudjeka I, Shen S, Liu Q, Whitney S, Keen T, Nair BC, Kalyanaraman VS, Markham P, Lu S. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology. 2006;350(1):34–47. doi: 10.1016/j.virol.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Zhan X, Martin LN, Slobod KS, Coleclough C, Lockey TD, Brown SA, Stambas J, Bonsignori M, Sealy RE, Blanchard JL, Hurwitz JL. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005;23(4647):5306–20. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]