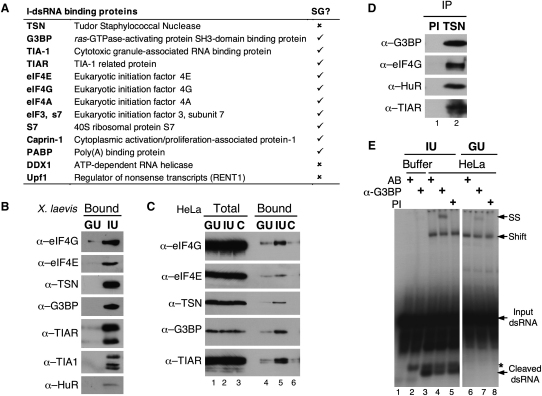
Figure 1.
A “Stress-Protein Complex” Forms Specifically on I-dsRNA
(A) Proteins that bind specifically to I-dsRNA. Previous characterization as an SG component is indicated.
(B) An immunoblot comprising X. laevis proteins eluted from GU and IU affinity matrices was probed with various antibodies.
(C) Immunoblots were used to analyze proteins bound to GU, IU, and C dsRNAs in HeLa lysates (lanes 4–6). Total protein is also shown (lanes 1–3).
(D) α-TSN IPs from HeLa lysates were analyzed by immunoblotting with antibodies against SG proteins. Preimmune serum (PI) was used as a control.
(E) When IU dsRNA was incubated with HeLa lysate, an RNA-protein complex was detected using EMSA (Shift; lanes 3–5). Cleaved I-dsRNA and a nonspecific cleavage product (∗) were also observed. A super-shifted (SS) complex was seen when α-G3BP was added (lane 4), but not with antibody buffer (AB) or PI (lanes 3 and 5, respectively). A GU RNA-protein complex also formed in HeLa lysate (lanes 6–8), and addition of α-G3BP resulted in a super-shifted complex (lane 7). This was absent with either AB or PI (lanes 6 and 8, respectively).