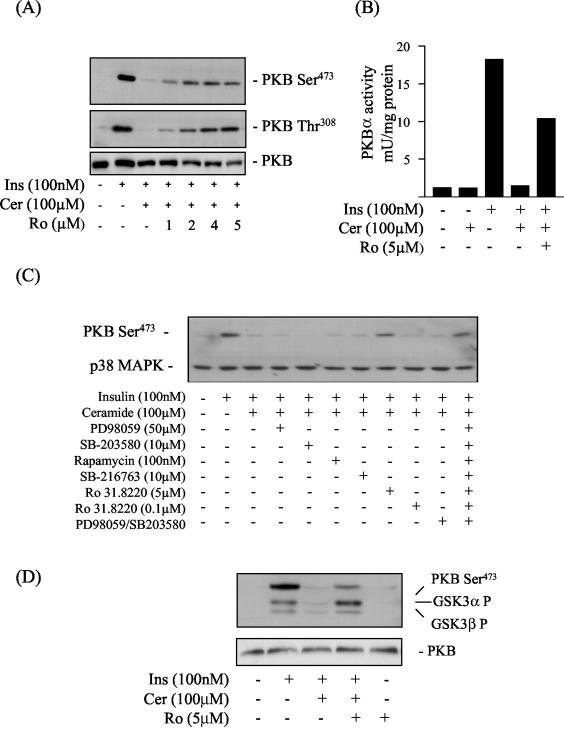
FIG. 1.
Representative blots showing the effects of ceramide and kinase inhibitors on the insulin-mediated phosphorylation of PKB and GSK3. L6 myotubes were incubated in the absence or presence of C2-ceramide (Cer, 100 μM) for 2 h. In some experiments, cells were preincubated with Ro 31.8220 (Ro, at the concentrations indicated) for 30 min prior to incubation with ceramide. At the end of this incubation period cells were incubated with insulin (Ins, 100 nM) for a further 10 min before being lysed. (A and B) Cell lysates were then immunoblotted with a phospho-specific antibody directed against PKB-Ser473, PKB-Thr308, or PKB (A) and used for assaying PKB activity as described in the text (B). (C) L6 myotubes were incubated in the absence or presence (singularly or in combination) of kinase inhibitors at the indicated concentrations for 30 min prior to incubation with C2-ceramide (100 μM) for 2 h and with insulin (100 nM) for 10 min. Cell lysates were immunoblotted with a phospho-specific antibody directed against PKB-Ser473, with blots being reprobed with an antibody against p38 MAPK (which was used as a marker for protein loading). (D) L6 myotubes were pretreated with Ro 31.8220 (Ro, 5 μM) for 30 min prior to incubation with C2-ceramide (100 μM) for 2 h and insulin (Ins, 100 nM) for 10 min. Cell lysates were immunoblotted with phospho-specific antibodies directed against either GSK3α-Ser21, GSK3β-Ser7, PKB-Ser473, or PKB.