FIG. 6.
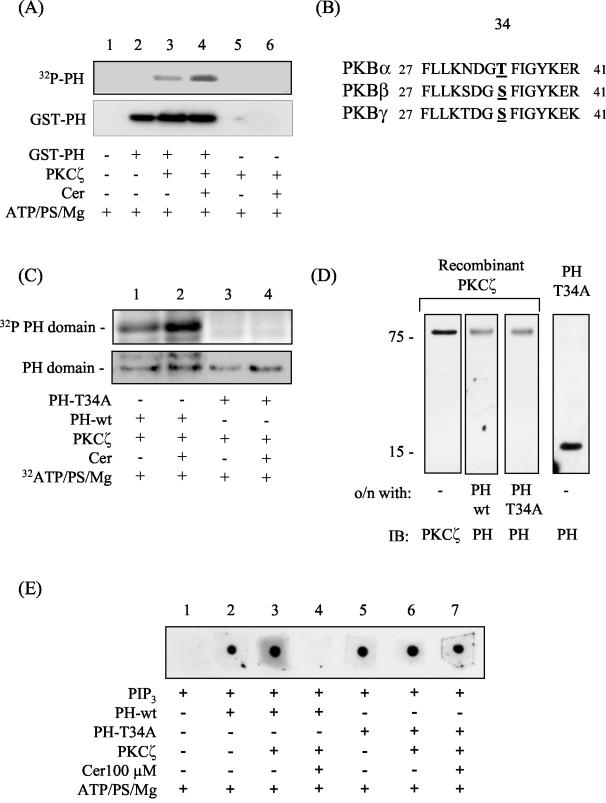
In vitro activation of PKCζ by ceramide results in the phosphorylation of the isolated PH domain of PKB on Thr34 with important consequences for PIP3 binding. (A) The isolated PH domain of PKB (1 μg) was incubated in the absence or presence of 30 ng of PKCζ and/or C2-ceramide (Cer, 100 μM) in buffer containing [γ-32P]ATP and cofactors required to support kinase activation for 20 min at 30°C as described in the text. Phosphorylated proteins were then resolved by SDS-PAGE and transferred to PVDF membranes prior to autoradiography and immunoblotting with anti-PH antibodies. (B) To identify putative PKCζ phosphorylation sites within the isolated PH domain of all three PKBisoforms, a web-based motif-scanning analysis tool was used (49). The aligned peptide sequences of all three PKB isoforms are shown and highlight the putative PKCζ phosphorylation site. (C) In vitro phosphorylation of wild-type (PH-wt) and T34A mutant PH (PH-T34A) domains was performed by incubating 30 ng of recombinant PKCζ with the appropriate PH peptide (1 μg of protein) in the presence of 1 μΜ [γ-32P]ATP and cofactors required for supporting kinase activation but in the absence or presence of C2-ceramide (Cer, 100 μM) for 20 min at 30°C as indicated. PH peptides were then resolved by SDS-PAGE and subjected to analysis by autoradiography. Protein loading was subsequently assessed with an antibody directed against the PKB-PH domain. (D) Far-Western analysis was performed by resolving recombinant PKCζ (0.1 μg of protein) by SDS-PAGE and transferring it onto PVDF membranes. The membranes were then incubated overnight at 4°C with either 0.1 μg of PH-wt (lane 2) or 0.1 μg of PH-T34A peptide (lane 3) prior to immunoblotting them with antibodies to PKCζ (lane 1) or the PH domain of PKB (lanes 2 to 4). (E) Protein-lipid overlay was performed to assess PIP3 binding to the PH-wt and T34A-PH peptides. Nitrocellulose membranes were spotted with 1 μl of PIP3 and subsequently incubated overnight at 4°C in TBST buffer containing 1 μM ATP, 4 pg of PS/ml, and 5 mM MgCl2. The incubation buffer either contained or lacked 0.5 μg of the appropriate PH peptide/ml, 0.5 μg of PKCζ/ml, and C2-ceramide (Cer, 100 μM) as indicated. Membranes were washed, and bound PH protein was detected by probing samples with an anti-PH domain antibody.