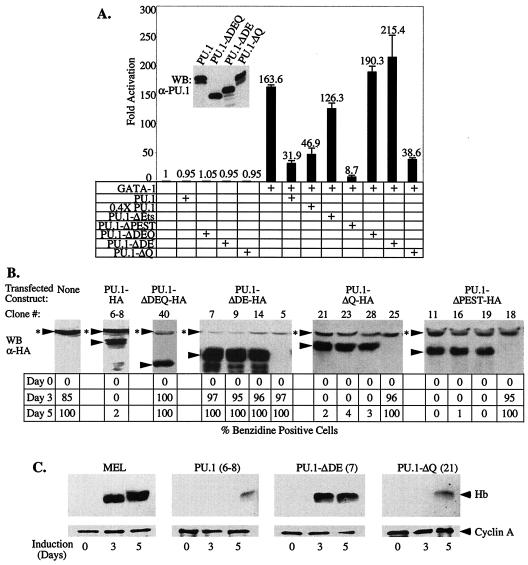
FIG. 6.
The acidic subdomain of PU.1 is required for repression of GATA-1 and inhibition of MEL cell differentiation. (A) U2OS cells were transfected with the αD3-Luc reporter (15 ng), pXM-GATA-1 (50 ng), and 100 ng of pEBB-PU.1 expression constructs or empty pEBB vector, as indicated. Luciferase activity was determined 48 h after transfection. (Inset) The expression level of the indicated proteins was analyzed by Western blotting (WB) with an anti-PU.1 antibody. (B) Stably transfected MEL cells clones expressing the indicated HA epitope-tagged PU.1 proteins were generated as described in Materials and Methods. The levels of transfected proteins were determined by Western blot analysis (WB) with an anti-HA antibody and compared to previously described tranfectants expressing wild-type PU.1-HA (clone 6-8) and PU.1-ΔDEQ-HA (50). Three clones with representative expression levels are shown for each type of expression construct. Arrowheads mark the position of specifically immunodetected proteins; arrowheads with asterisks indicate the position of a nonspecific band detected with the anti-HA antibody. The clones were treated with 1.8% DMSO and, at the indicated times, the percentage of hemoglobinized, benzidine-positive cells was determined. Similar results were obtained with three to five additional clones for each type of transfected construct. (C) Cell extracts of the indicated transfectants (clone number in parenthesis) were prepared after 3 and 5 days of treatment with 1.8% DMSO and from untreated cells (day 0). Equal amounts of protein were loaded in each lane and hemoglobin (Hb) levels were determined by Western blot analysis with an anti-Hb antibody. An anti-cyclin A antibody was used as a loading control. A schematic diagram of the mutant PU.1 proteins is shown in Fig. 4D.