Abstract
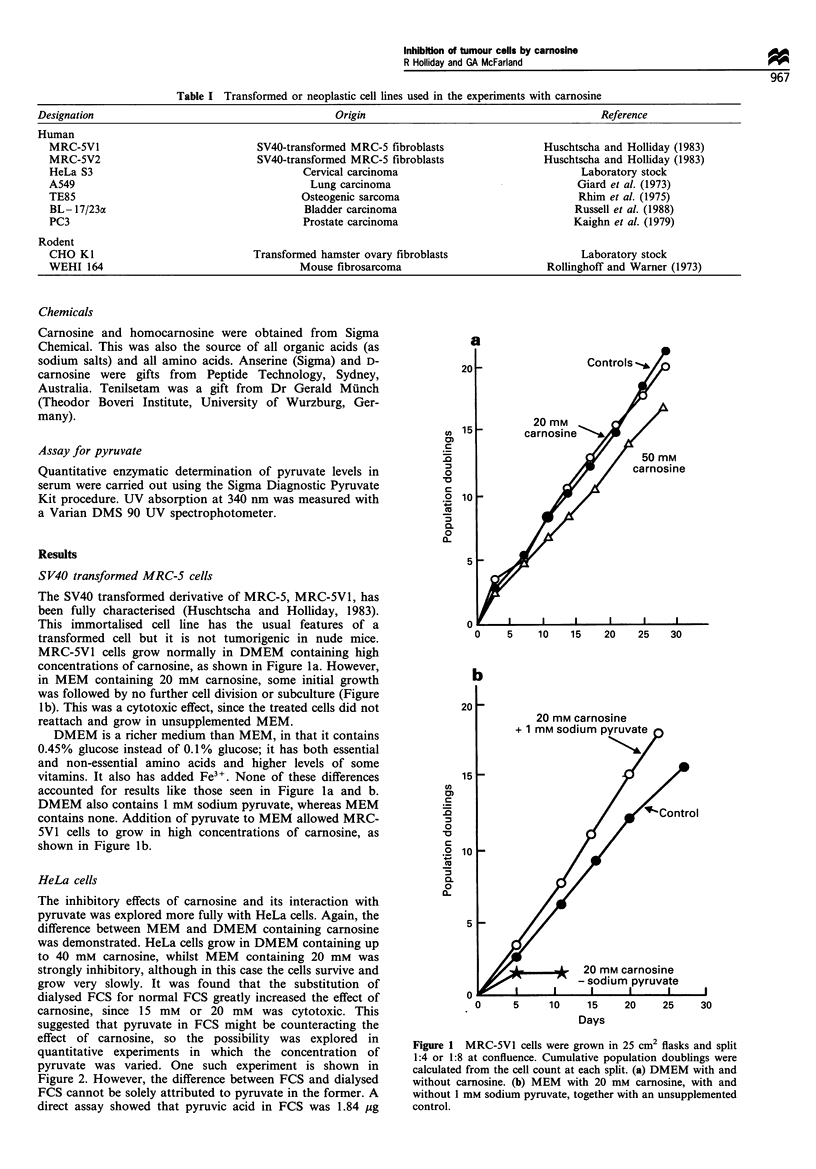
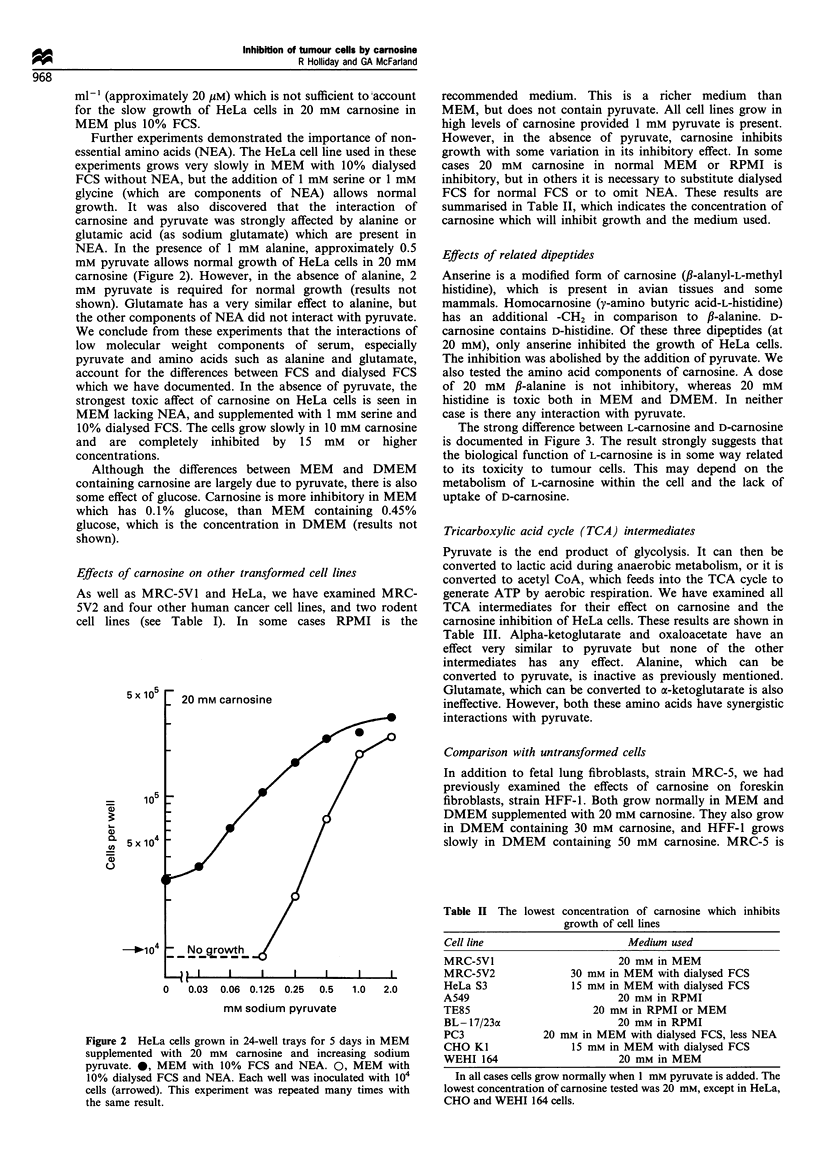
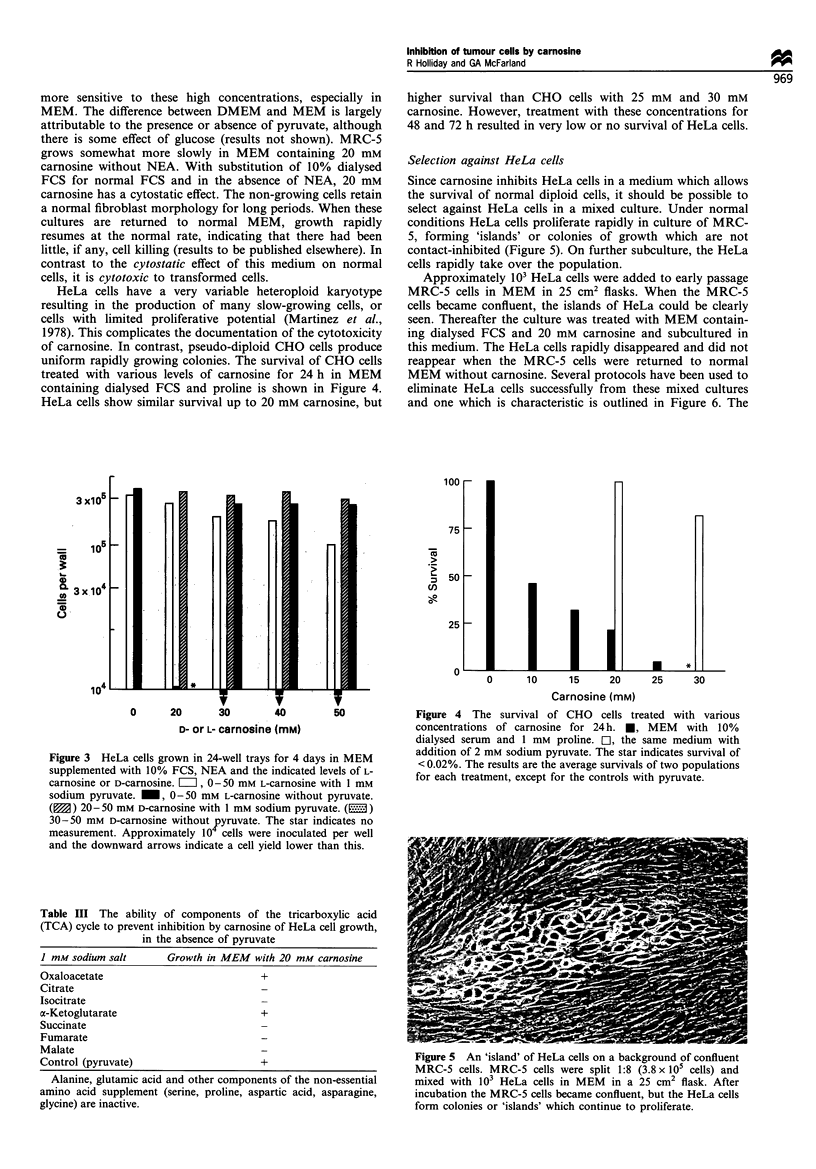
Human diploid fibroblasts growth normally in medium containing physiological concentrations of the naturally occurring dipeptide carnosine (beta-alanyl-L-histidine). These concentrations are cytotoxic to transformed and neoplastic cells lines in modified Eagle medium (MEM), whereas these cells grow vigorously in Dulbecco's modified Eagle medium (DMEM) containing carnosine. This difference is due to the presence of 1 mM sodium pyruvate in DMEM. Seven human cell lines and two rodent cell lines were tested and all are strongly inhibited by carnosine in the absence of pyruvate. Experiments with HeLa cells show that anserine is similar to carnosine, but D-carnosine and homocarnosine are without effect. Also, the non-essential amino acids alanine and glutamic acid contribute to the effect of pyruvate in preventing carnosine toxicity, and oxaloacetate and alpha-ketoglutarate can substitute for pyruvate. We have used mixtures of normal MRC-5 fibroblasts and HeLa cells to demonstrate that 20 mM carnosine can selectively eliminate the tumour cells. This has obvious implications which might be exploited in in vivo and in vitro studies. Carnosine is known to react strongly with aldehyde and keto groups of sugars by Amadori reaction, and we propose that it depletes certain glycolysis intermediates. It is well known that tumour cells are more dependent on glycolysis than normal cells. A reduction of glycolysis intermediates by carnosine may deplete their energy supply, but this effect is totally reversed by pyruvate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Hipkiss A. R., Michaelis J., Syrris P., Kumar S., Lam Y. Carnosine protects proteins against in vitro glycation and cross-linking. Biochem Soc Trans. 1994 Nov;22(4):399S–399S. doi: 10.1042/bst022399s. [DOI] [PubMed] [Google Scholar]
- Hipkiss A. R., Michaelis J., Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995 Aug 28;371(1):81–85. doi: 10.1016/0014-5793(95)00849-5. [DOI] [PubMed] [Google Scholar]
- Huschtscha L. I., Holliday R. Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J Cell Sci. 1983 Sep;63:77–99. doi: 10.1242/jcs.63.1.77. [DOI] [PubMed] [Google Scholar]
- Kaighn M. E., Narayan K. S., Ohnuki Y., Lechner J. F., Jones L. W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979 Jul;17(1):16–23. [PubMed] [Google Scholar]
- Mannion A. F., Jakeman P. M., Dunnett M., Harris R. C., Willan P. L. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur J Appl Physiol Occup Physiol. 1992;64(1):47–50. doi: 10.1007/BF00376439. [DOI] [PubMed] [Google Scholar]
- Martinez A. O., Norwood T. H., Prothero J. W., Martin G. M. Evidence for clonal attenuation of growth potential in HeLa cells. In Vitro. 1978 Dec;14(12):996–1002. doi: 10.1007/BF02616213. [DOI] [PubMed] [Google Scholar]
- McFarland G. A., Holliday R. Retardation of the senescence of cultured human diploid fibroblasts by carnosine. Exp Cell Res. 1994 Jun;212(2):167–175. doi: 10.1006/excr.1994.1132. [DOI] [PubMed] [Google Scholar]
- Münch G., Taneli Y., Schraven E., Schindler U., Schinzel R., Palm D., Riederer P. The cognition-enhancing drug tenilsetam is an inhibitor of protein crosslinking by advanced glycosylation. J Neural Transm Park Dis Dement Sect. 1994;8(3):193–208. doi: 10.1007/BF02260940. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Boldyrev A. A., Formazuyk V. E. Carnosine: its properties, functions and potential therapeutic applications. Mol Aspects Med. 1992;13(5):379–444. doi: 10.1016/0098-2997(92)90006-l. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Russell P. J., Wotherspoon J., Jelbart M., Philips J., Raghavan D. Stability of lectin binding properties expressed by human bladder carcinoma cell lines passaged in vitro or in nude mice. Urol Res. 1988;16(6):407–414. doi: 10.1007/BF00280020. [DOI] [PubMed] [Google Scholar]
- Röllinghoff M., Warner N. L. Specificity of in vivo tumor rejection assessed by mixing immune spleen cells with target and unrelated tumor cells. Proc Soc Exp Biol Med. 1973 Dec;144(3):813–818. doi: 10.3181/00379727-144-37688. [DOI] [PubMed] [Google Scholar]
- Shapot V. S. Some biochemical aspects of the relationship between the tumor and the host. Adv Cancer Res. 1972;15:253–286. doi: 10.1016/s0065-230x(08)60377-2. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]



