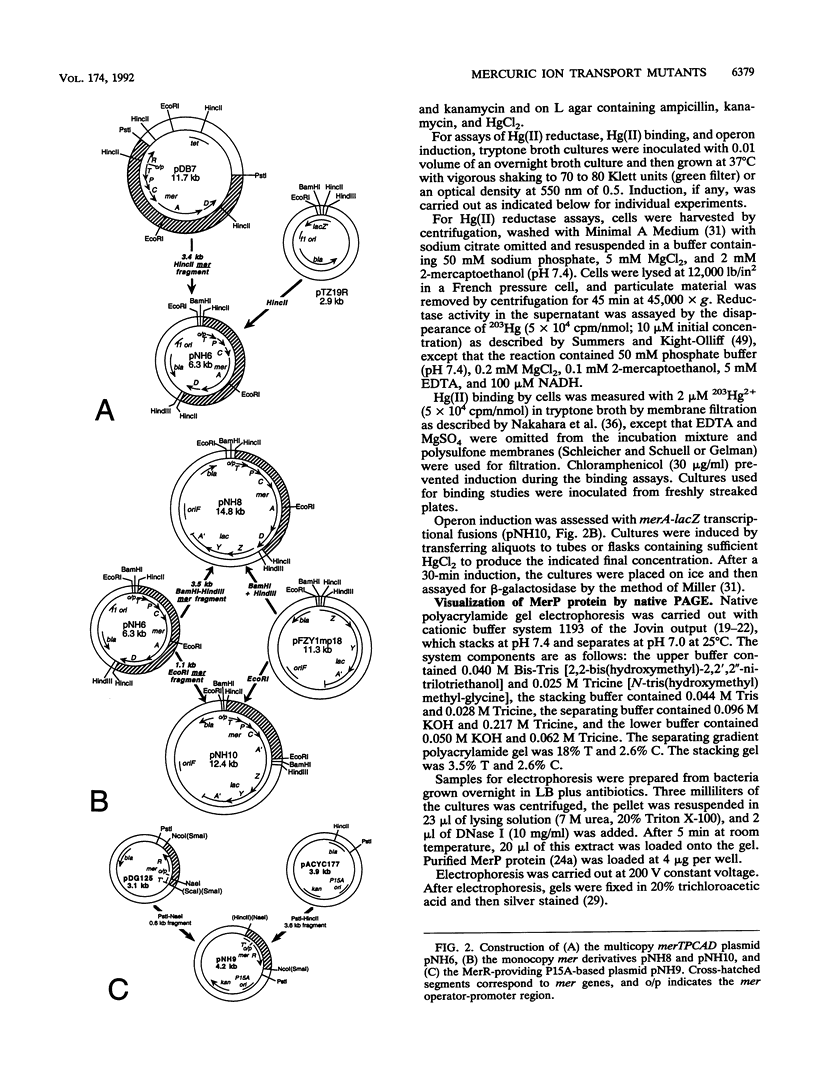
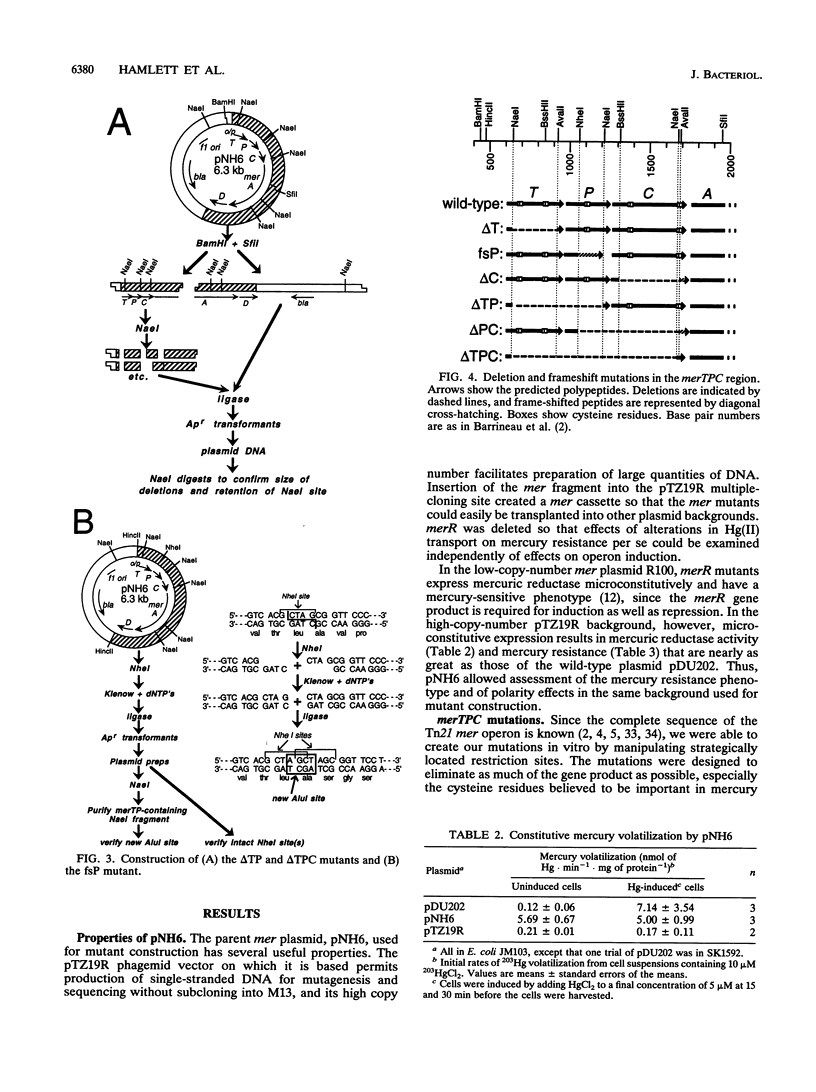
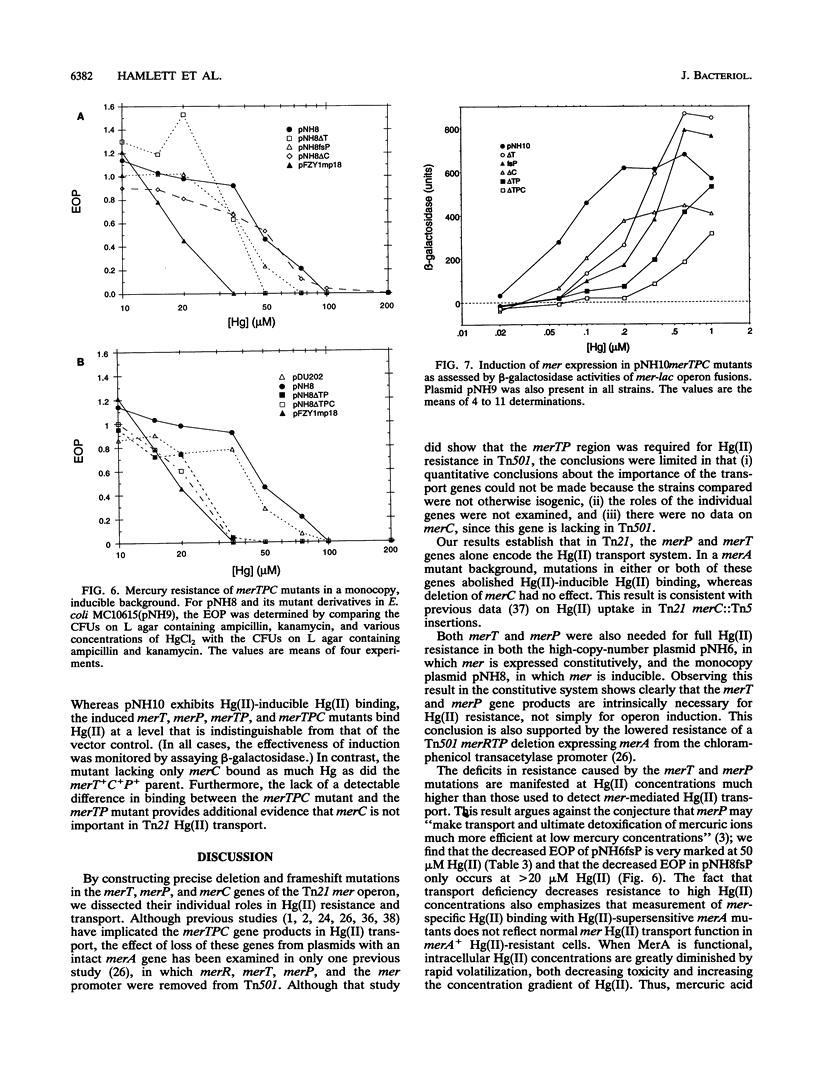
Abstract
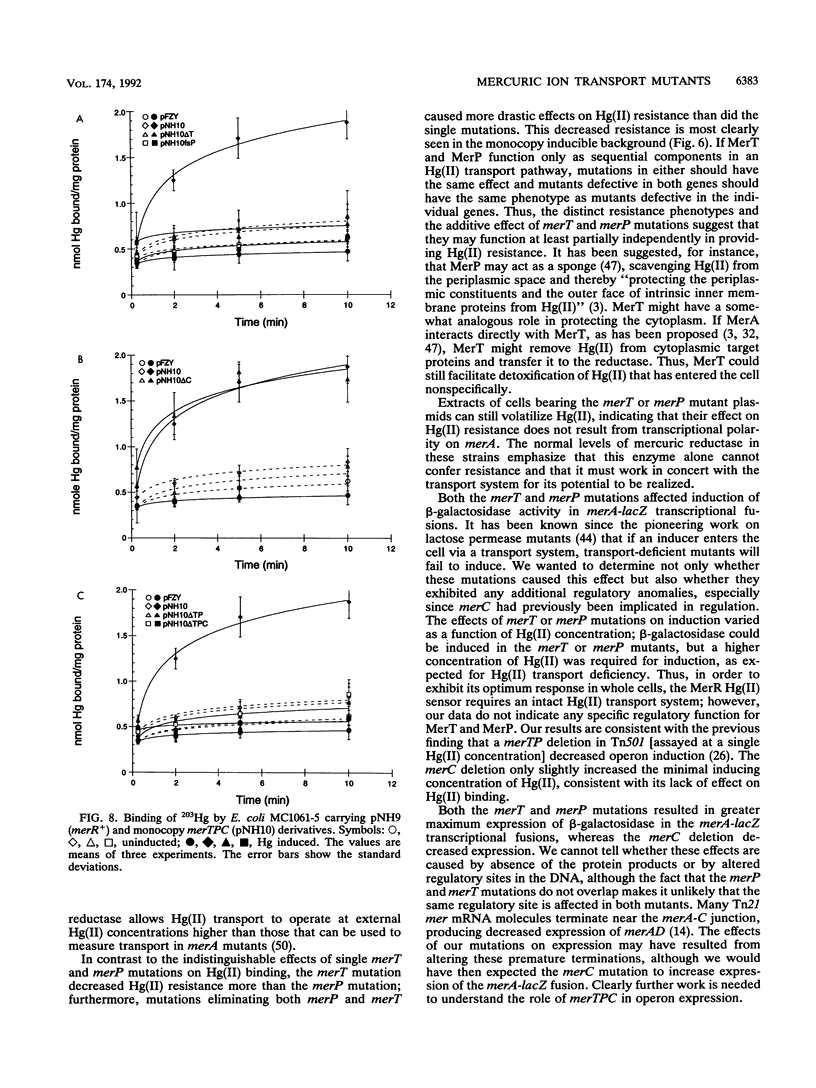
The mercury resistance (mer) operon of the gram-negative transposon Tn21 encodes not only a mercuric reductase and regulatory genes but also two inner membrane proteins (MerT and MerC) and a periplasmic protein (MerP). Although the merT, merP, and merC genes have been implicated in Hg(II) transport, the individual roles of these genes have not been established. We created in vitro precise deletion and frameshift mutations that eliminated each of the genes singly and in combination. Our results show that both merT and merP are required for Hg(II) binding but that merC is not. Both merT and merP are required for full expression of Hg(II) resistance, but loss of merP is less deleterious than loss of merT. Furthermore, mutations eliminating both merT and merP decrease resistance more than the single mutations do. In contrast, mutating merC had no effect on Hg(II) resistance. Both the merT and merP mutations increase the threshold Hg(II) concentration for induction of merA-lacZ transcriptional fusions and cause an increase in the maximal expression level. In contrast, the merC mutation had little effect on the threshold inducing concentration of Hg(II) but decreased the level of expression. Our results show that merT and merP alone are sufficient to specify a mercury transport system. The role of merC remains obscure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Barrineau P., Gilbert P., Jackson W. J., Jones C. S., Summers A. O., Wisdom S. The DNA sequence of the mercury resistance operon of the IncFII plasmid NR1. J Mol Appl Genet. 1984;2(6):601–619. [PubMed] [Google Scholar]
- Barrineau P., Summers A. O. A second positive regulatory function in the mer (mercury resistance) operon. Gene. 1983 Nov;25(2-3):209–221. doi: 10.1016/0378-1119(83)90225-1. [DOI] [PubMed] [Google Scholar]
- Bhriain N. N., Foster T. J. Polypeptides specified by the mercuric resistance (mer) operon of plasmid R100. Gene. 1986;42(3):323–330. doi: 10.1016/0378-1119(86)90236-2. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Camakaris J., Lee B. T., Williams T., Morby A. P., Parkhill J., Rouch D. A. Bacterial resistances to mercury and copper. J Cell Biochem. 1991 Jun;46(2):106–114. doi: 10.1002/jcb.240460204. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Ford S. J., Pridmore R. D., Fritzinger D. C. Nucleotide sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry. 1983 Aug 16;22(17):4089–4095. doi: 10.1021/bi00286a015. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Misra T. K., Winnie J. N., Schmidt A., Seiff M., Silver S. The nucleotide sequence of the mercuric resistance operons of plasmid R100 and transposon Tn501: further evidence for mer genes which enhance the activity of the mercuric ion detoxification system. Mol Gen Genet. 1986 Jan;202(1):143–151. doi: 10.1007/BF00330531. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Brown N. L. Identification of the merR gene of R100 by using mer-lac gene and operon fusions. J Bacteriol. 1985 Sep;163(3):1153–1157. doi: 10.1128/jb.163.3.1153-1157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Ginnity F. Some mercurial resistance plasmids from different incompatibility groups specify merR regulatory functions that both repress and induce the mer operon of plasmid R100. J Bacteriol. 1985 May;162(2):773–776. doi: 10.1128/jb.162.2.773-776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Insertion of the tetracycline resistance translocation unit Tn10 in the lac operon of Escherichia coli K12. Mol Gen Genet. 1977 Sep 9;154(3):305–309. doi: 10.1007/BF00571287. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Nakahara H., Weiss A. A., Silver S. Transposon A-generated mutations in the mercuric resistance genes of plasmid R100-1. J Bacteriol. 1979 Oct;140(1):167–181. doi: 10.1128/jb.140.1.167-181.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambill B. D., Summers A. O. Synthesis and degradation of the mRNA of the Tn21 mer operon. J Mol Biol. 1992 May 20;225(2):251–259. doi: 10.1016/0022-2836(92)90919-b. [DOI] [PubMed] [Google Scholar]
- Gilbert M. P., Summers A. O. The distribution and divergence of DNA sequences related to the Tn21 and Tn501 mer operons. Plasmid. 1988 Sep;20(2):127–136. doi: 10.1016/0147-619x(88)90015-7. [DOI] [PubMed] [Google Scholar]
- Heltzel A., Gambill D., Jackson W. J., Totis P. A., Summers A. O. Overexpression and DNA-binding properties of the mer-encoded regulatory protein from plasmid NR1 (Tn21). J Bacteriol. 1987 Jul;169(7):3379–3384. doi: 10.1128/jb.169.7.3379-3384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Kusano T. The merR regulatory gene in Thiobacillus ferrooxidans is spaced apart from the mer structural genes. Mol Microbiol. 1991 Nov;5(11):2707–2718. doi: 10.1111/j.1365-2958.1991.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Jackson W. J., Summers A. O. Biochemical characterization of HgCl2-inducible polypeptides encoded by the mer operon of plasmid R100. J Bacteriol. 1982 Aug;151(2):962–970. doi: 10.1128/jb.151.2.962-970.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis. 3. Further analysis and new forms of discontinuous buffer systems. Biochemistry. 1973 Feb 27;12(5):890–898. doi: 10.1021/bi00729a016. [DOI] [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis. I. Steady-state moving-boundary systems formed by different electrolyte combinations. Biochemistry. 1973 Feb 27;12(5):871–879. doi: 10.1021/bi00729a014. [DOI] [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis. II. Design of integrated discontinuous buffer systems for analytical and preparative fractionation. Biochemistry. 1973 Feb 27;12(5):879–890. doi: 10.1021/bi00729a015. [DOI] [PubMed] [Google Scholar]
- Koop A. H., Hartley M. E., Bourgeois S. A low-copy-number vector utilizing beta-galactosidase for the analysis of gene control elements. Gene. 1987;52(2-3):245–256. doi: 10.1016/0378-1119(87)90051-5. [DOI] [PubMed] [Google Scholar]
- Kusano T., Ji G. Y., Inoue C., Silver S. Constitutive synthesis of a transport function encoded by the Thiobacillus ferrooxidans merC gene cloned in Escherichia coli. J Bacteriol. 1990 May;172(5):2688–2692. doi: 10.1128/jb.172.5.2688-2692.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. W., Gambill B. D., Summers A. O. Translation of merD in Tn21. J Bacteriol. 1989 Apr;171(4):2222–2225. doi: 10.1128/jb.171.4.2222-2225.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. A., Brown N. L. Role of the merT and merP gene products of transposon Tn501 in the induction and expression of resistance to mercuric ions. Gene. 1987;52(2-3):207–214. doi: 10.1016/0378-1119(87)90047-3. [DOI] [PubMed] [Google Scholar]
- Lund P. A., Ford S. J., Brown N. L. Transcriptional regulation of the mercury-resistance genes of transposon Tn501. J Gen Microbiol. 1986 Feb;132(2):465–480. doi: 10.1099/00221287-132-2-465. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K. Bacterial resistances to inorganic mercury salts and organomercurials. Plasmid. 1992 Jan;27(1):4–16. doi: 10.1016/0147-619x(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Fritzinger D. C., Pridmore R. D., Barnes W. M., Haberstroh L., Silver S. Mercuric ion-resistance operons of plasmid R100 and transposon Tn501: the beginning of the operon including the regulatory region and the first two structural genes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5975–5979. doi: 10.1073/pnas.81.19.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Haberstroh L., Schmidt A., Goddette D., Silver S. Mercuric reductase structural genes from plasmid R100 and transposon Tn501: functional domains of the enzyme. Gene. 1985;34(2-3):253–262. doi: 10.1016/0378-1119(85)90134-9. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D., Yu H. R., Nucifora G., Misra T. K. Purification and functional characterization of MerD. A coregulator of the mercury resistance operon in gram-negative bacteria. J Biol Chem. 1991 Oct 5;266(28):18538–18542. [PubMed] [Google Scholar]
- Nakahara H., Silver S., Miki T., Rownd R. H. Hypersensitivity to Hg2+ and hyperbinding activity associated with cloned fragments of the mercurial resistance operon of plasmid NR1. J Bacteriol. 1979 Oct;140(1):161–166. doi: 10.1128/jb.140.1.161-166.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni'Bhriain N. N., Silver S., Foster T. J. Tn5 insertion mutations in the mercuric ion resistance genes derived from plasmid R100. J Bacteriol. 1983 Aug;155(2):690–703. doi: 10.1128/jb.155.2.690-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Silver S., Misra T. K. Down regulation of the mercury resistance operon by the most promoter-distal gene merD. Mol Gen Genet. 1989 Dec;220(1):69–72. doi: 10.1007/BF00260858. [DOI] [PubMed] [Google Scholar]
- O'Halloran T., Walsh C. Metalloregulatory DNA-binding protein encoded by the merR gene: isolation and characterization. Science. 1987 Jan 9;235(4785):211–214. doi: 10.1126/science.3798107. [DOI] [PubMed] [Google Scholar]
- Silver S., Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992 Mar;56(1):195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Kight-Olliff L. Tn1 generated mutants in the mercuric ion reductase of the Inc P plasmid, R702. Mol Gen Genet. 1980;180(1):91–97. doi: 10.1007/BF00267356. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Knight-Olliff L., Slater C. Effect of catabolite repression on the mer operon. J Bacteriol. 1982 Jan;149(1):191–197. doi: 10.1128/jb.149.1.191-197.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Summers A. O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992 May;174(10):3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]