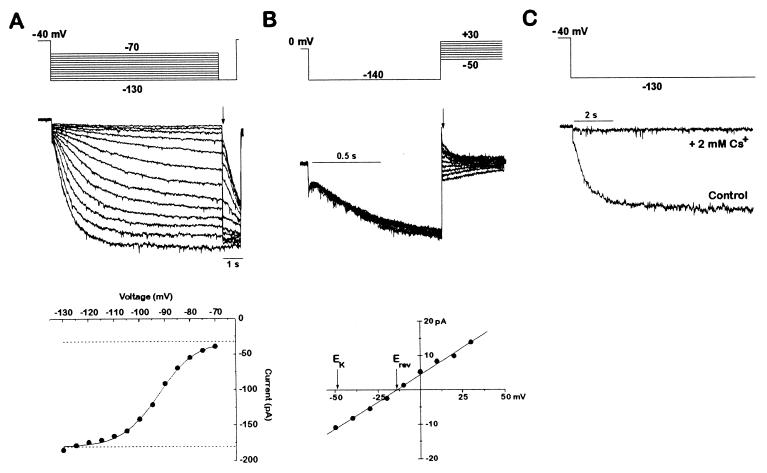
Figure 2.
A hyperpolarization-activated Ih current is present in zebrafish cardiac myocytes. (A) Activation of Ih current by hyperpolarization. An individual dissociated cardiomyocyte was voltage-clamped from a holding potential of −40 mV to various hyperpolarizing voltages (−70 to −130 mV in 10-mV increments) once every 15 s. Each hyperpolarization was followed by a step to −130 mV to determine the current at a constant voltage. The current amplitude immediately after the voltage step to −130 mV was then plotted as a function of the activation voltage. The data were fitted with a Boltzmann function. The amplitude of the Ih current was given by the net amplitude of the Boltzmann function. The leak current was determined by the baseline of the fit and was not part of the Ih determination. Current traces were filtered at 1 kHz. (B) Tail current analysis of the Ih reversal potential. After Ih was activated for 1 s by a step from 0 mV to −130 mV, another step was applied to a new voltage (from −50 to +30 mV in 10-mV increments). The tail current amplitude immediately after the voltage clamp settled is plotted as a function of the tail current voltage. The tail currents reversed at −14 mV, as determined by the line fitted to the data points. A perfectly potassium-selective channel would have a reversal potential of −49 mV in the solutions used. The dihydropyridine niguldipine (1 μM) was included to block the calcium currents present in these cells. If the cell was held at 0 mV and not hyperpolarized to activate the Ih current, no tail currents were observed (data not shown). Current traces were filtered at 2 kHz. The voltage protocol was delivered once every 4 s. (C) Inhibition of Ih by cesium. Ih currents with and without 2 mM CsCl applied extracellularly, in response to a step from −40 mV to −130 mV. Current traces were filtered at 1 kHz.