Abstract
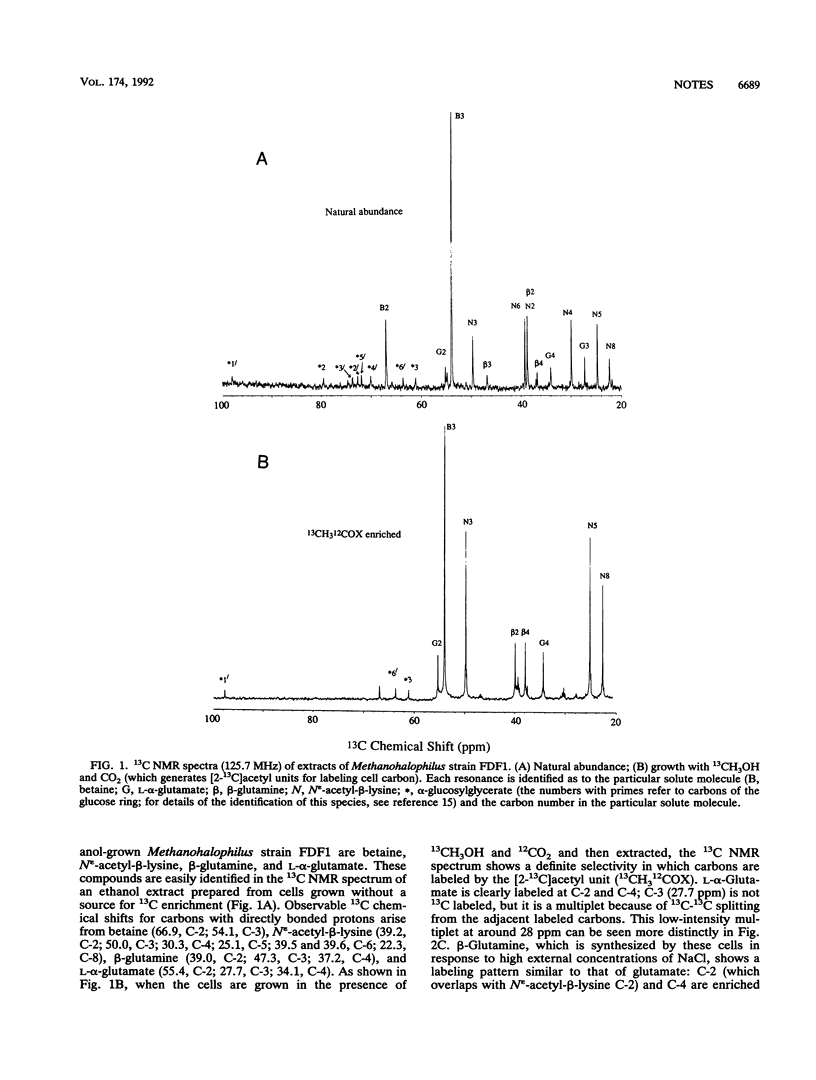
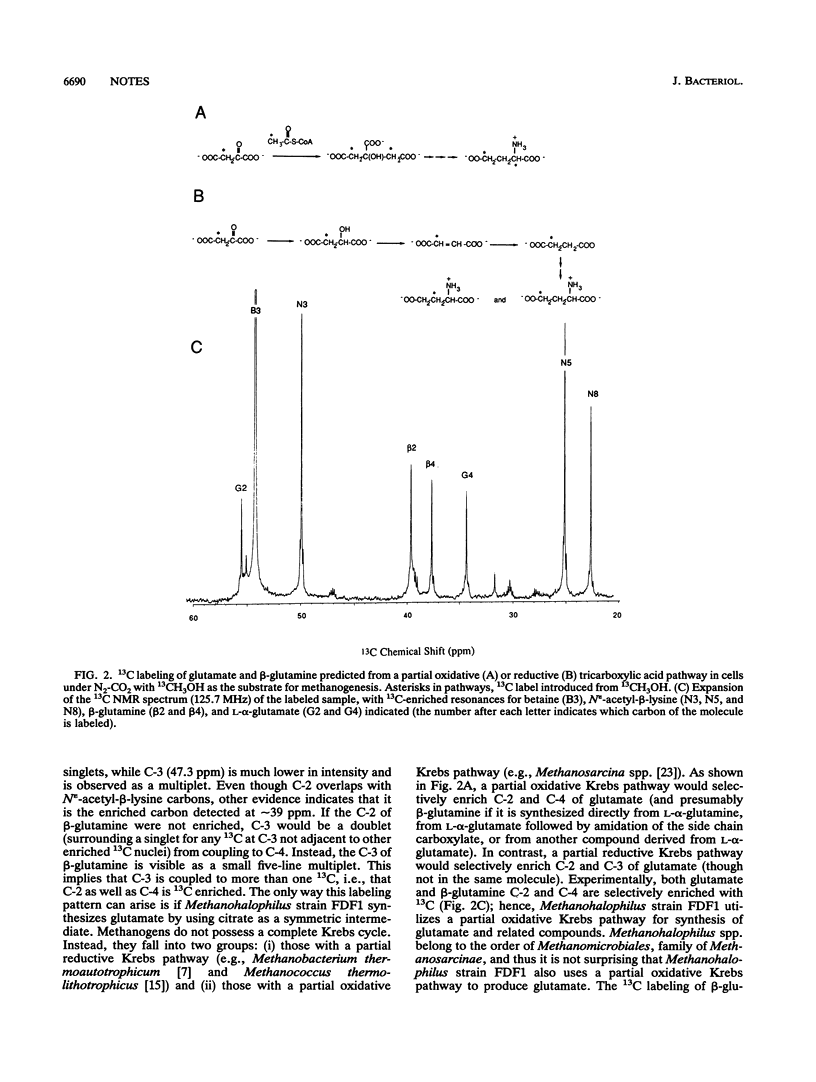
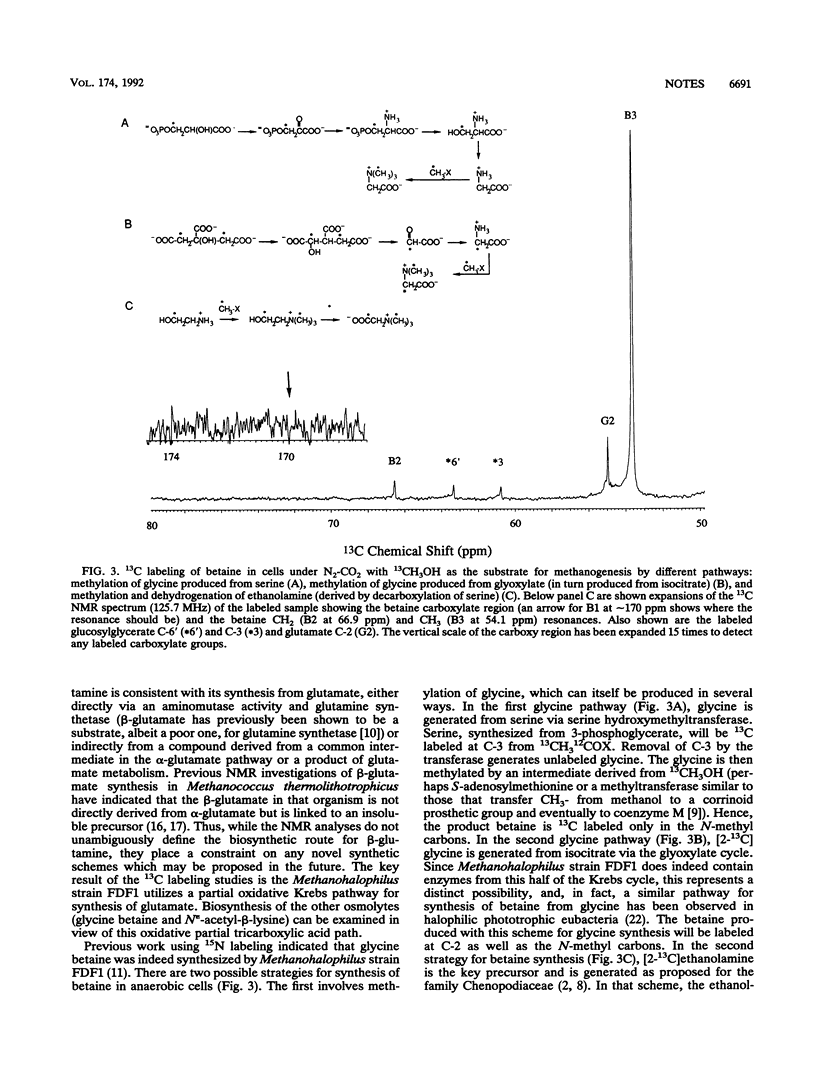
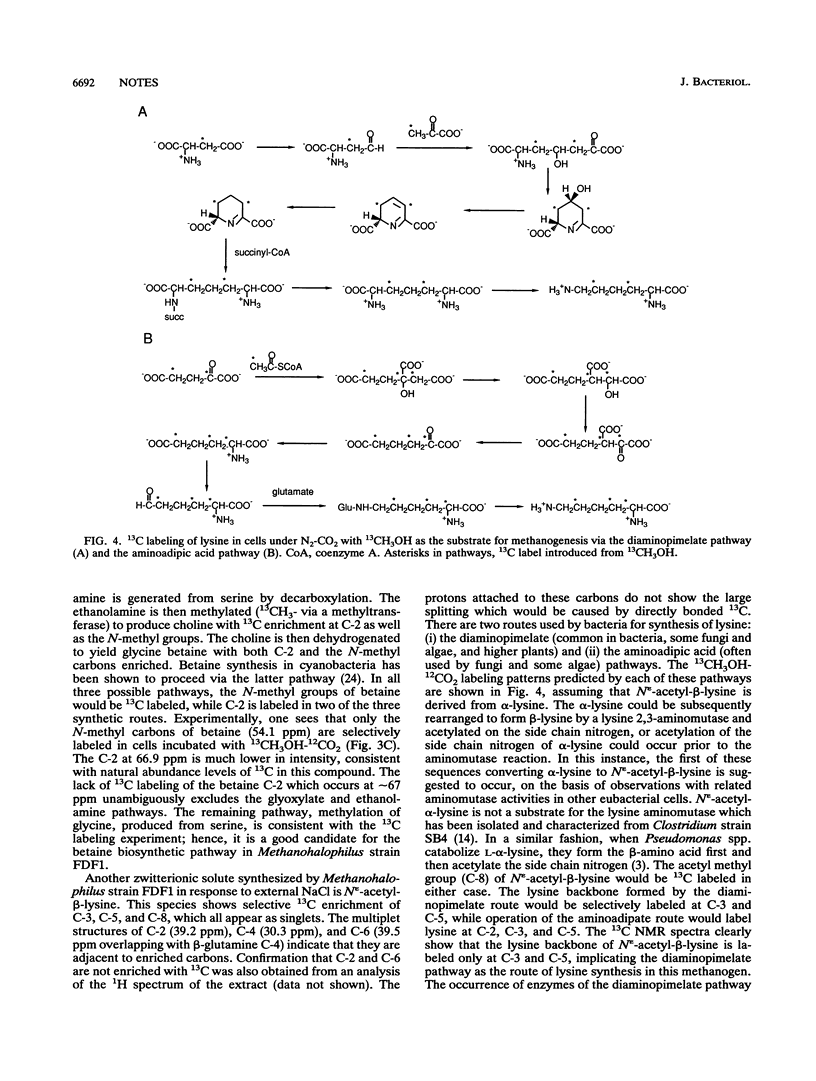
Methanohalophilus strain FDF1 synthesizes beta-glutamine, betaine, and N epsilon-acetyl-beta-lysine as osmoprotective agents when the cells are grown in high external concentrations of NaCl. Nuclear magnetic resonance spectroscopic analyses of 13CH3OH-12CO2 label incorporation by the cells provide information on the biosynthetic pathways of these organic osmolytes. The labeling studies indicate that Methanohalophilus strain FDF1 produces glutamate and beta-glutamine via a partial oxidative Krebs pathway. 13C labeling of betaine is consistent with methylation of glycine generated from serine (via serine hydroxymethyltransferase). The labeling pattern for N epsilon-acetyl-beta-lysine is consistent with the synthesis of its precursor alpha-lysine occurring by the diaminopimelate pathway in these cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baraniak J., Moss M. L., Frey P. A. Lysine 2,3-aminomutase. Support for a mechanism of hydrogen transfer involving S-adenosylmethionine. J Biol Chem. 1989 Jan 25;264(3):1357–1360. [PubMed] [Google Scholar]
- Edmunds H. N., Barker H. A. Aerobic metabolism of L- -lysine in a Pseudomonas. Coenzyme A-dependent acetylation of L- -lysine. Arch Biochem Biophys. 1973 Jan;154(1):460–470. doi: 10.1016/0003-9861(73)90079-9. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Smith I. C., Sprott G. D. Biosynthetic pathways in Methanospirillum hungatei as determined by 13C nuclear magnetic resonance. J Bacteriol. 1983 Oct;156(1):316–326. doi: 10.1128/jb.156.1.316-326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. N., Tolman C. J., Kanodia S., Roberts M. F. 2,3-Cyclopyrophosphoglycerate in methanogens: evidence by 13C NMR spectroscopy for a role in carbohydrate metabolism. Biochemistry. 1985 Oct 8;24(21):5693–5698. doi: 10.1021/bi00342a001. [DOI] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E. Evidence for an incomplete reductive carboxylic acid cycle in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Jul;118(1):121–125. doi: 10.1007/BF00406084. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., May A. M., Grumet R., Bode J., Jamieson G. C., Rhodes D. Betaine synthesis in chenopods: Localization in chloroplasts. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3678–3682. doi: 10.1073/pnas.82.11.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHEDOURI E., MEISTER A. SYNTHESIS OF D-BETA-GLUTAMINE FROM BETA-GLUTAMIC ACID BY GLUTAMINE SYNTHETASE. J Biol Chem. 1965 Aug;240:3357–3360. [PubMed] [Google Scholar]
- Lai M. C., Sowers K. R., Robertson D. E., Roberts M. F., Gunsalus R. P. Distribution of compatible solutes in the halophilic methanogenic archaebacteria. J Bacteriol. 1991 Sep;173(17):5352–5358. doi: 10.1128/jb.173.17.5352-5358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathrani I. M., Boone D. R. Isolation and characterization of a moderately halophilic methanogen from a solar saltern. Appl Environ Microbiol. 1985 Jul;50(1):140–143. doi: 10.1128/aem.50.1.140-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Lai M. C., Gunsalus R. P., Roberts M. F. Composition, Variation, and Dynamics of Major Osmotic Solutes in Methanohalophilus Strain FDF1. Appl Environ Microbiol. 1992 Aug;58(8):2438–2443. doi: 10.1128/aem.58.8.2438-2443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Lesage S., Roberts M. F. Beta-aminoglutaric acid is a major soluble component of Methanococcus thermolithotrophicus. Biochim Biophys Acta. 1989 Sep 15;992(3):320–326. doi: 10.1016/0304-4165(89)90091-3. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Noll D., Roberts M. F. Free amino acid dynamics in marine methanogens. beta-Amino acids as compatible solutes. J Biol Chem. 1992 Jul 25;267(21):14893–14901. [PubMed] [Google Scholar]
- Robertson D. E., Noll D., Roberts M. F., Menaia J. A., Boone D. R. Detection of the osmoregulator betaine in methanogens. Appl Environ Microbiol. 1990 Feb;56(2):563–565. doi: 10.1128/aem.56.2.563-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Roberts M. F., Belay N., Stetter K. O., Boone D. R. Occurrence of beta-glutamate, a novel osmolyte, in marine methanogenic bacteria. Appl Environ Microbiol. 1990 May;56(5):1504–1508. doi: 10.1128/aem.56.5.1504-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Roberts M. F. Organic osmolytes in methanogenic archaebacteria. Biofactors. 1991 Jan;3(1):1–9. [PubMed] [Google Scholar]
- Sowers K. R., Robertson D. E., Noll D., Gunsalus R. P., Roberts M. F. N epsilon-acetyl-beta-lysine: an osmolyte synthesized by methanogenic archaebacteria. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9083–9087. doi: 10.1073/pnas.87.23.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Usher J. R. The electrochemical proton gradient and phenylalanine transport in Escherichia coli irradiated with near-ultraviolet light. Can J Microbiol. 1977 Dec;23(12):1683–1688. doi: 10.1139/m77-242. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate assimilation pathway of Methanosarcina barkeri. J Bacteriol. 1979 Jan;137(1):332–339. doi: 10.1128/jb.137.1.332-339.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


