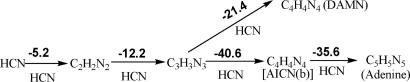
Fig. 2.
Thermochemistry of pentamerization of HCN. The relative energies in gas phase are in kilocalories per mole computed at B3LYP/6–311+G**+ZPVE. Entropy is unfavorable but is not included in each step. Overall energy for pentamerization of adenine (5 HCN → C5H5N5) is −93.8 kcal/mol (ΔG298 = −53.7 kcal/mol). Note that the last crucial step for formation of pentamer (adenine) from tetramer [AICN(b)] is highly exothermic.