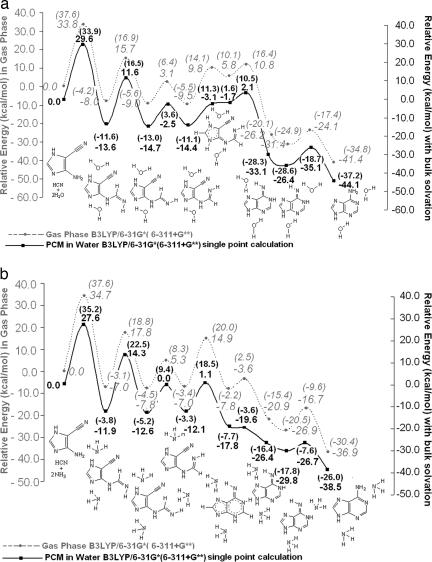
Fig. 6.
Reaction profiles for the formation of adenine starting from AICN(b) and HCN in the gas phase and with simulated bulk water solvation by means of explicit solvent-catalyzed mechanisms (two solvent molecules). (a) Reaction profile with water as explicit catalytic molecule (gas phase vs. simulated bulk water solvation). (b) Reaction profile with ammonia as explicit catalytic molecule (gas phase vs. simulated bulk water solvation). The two H2O or NH3 molecules facilitate a “proton relay” by forming an H-bonded “circuit” for the proton transfer in a six-membered transition state. All species shown are stable minima. (Dotted lines depict partial bonds in complexes or transition states.) The comparisons with the gas-phase profiles show the large extent to which simulated water solvation reduces the barrier electrostatically. The first step is rate-determining in all cases. The basis set dependency of the barrier heights is shown by the comparison data at 6-31G* and at 6-311+G** (in parentheses).