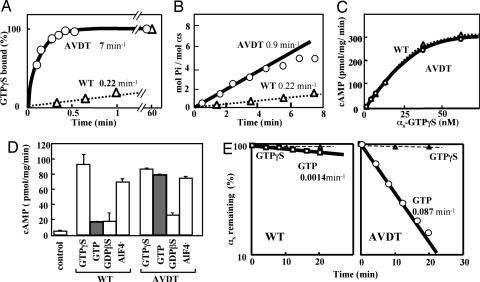
Fig. 2.
Biochemical properties of recombinant WT (αs-WT) and mutant (αs-AVDT) αs proteins. (A) Rates of GTP[γS] binding. Recombinant αs-WT (triangles) and αs-AVDT (circles) proteins (each at 40 nM) were incubated at 22°C with 1 μM [35S]GTP[γS] (2.2 × 105 cpm/pmol) in buffer A [20 mM Na-Hepes, pH 7.4/10 mM MgSO4/0.1 mM EDTA/3 mM 2-mercaptoethanol/0.025% polyoxyethylene (10) lauryl ether (C12E10)]. At the times indicated, the reaction was terminated, and GTP[γS] binding was quantitated by filtration on nitrocellulose membranes. The apparent on rates of GTP[γS] binding (kapp) were then calculated by fitting the data to the equation B = Beq (1 − e−kt) as described in Materials and Methods, where B is the concentration of bound GTP[γS], Beq is the equilibreated concentration of binding sites, k is the constant describing the rate of approach of the reaction to Beq, and t is time. (B) Steady-state GTPase. Forty nanomolar of recombinant αs-WT (triangles) or αs-AVDT (circles) was incubated for the indicated times at 22°C with 2 μM [γ-32P]GTP (2 × 105 cpm/pmol−1) in buffer A. At the times indicated, the reactions were terminated, and phosphate release was quantified by charcoal adsorption. The steady-state GTPase rates (kss) were then calculated. Pi, inorganic phosphate. (C) cAMP synthesis stimulated by different concentrations of αs-WT or αs-AVDT in the presence of GTP[γS]. Reactions were performed at 20°C for 10 min in 75-μl volumes containing 22.5 μg of cyc− cell membranes, as described previously (12, 47) apart from minor changes to the buffer conditions. These included 50 mM Na-Hepes (pH 7.4), 6 mM MgCl2, 0.01% C12E10, and 50 μg ml−1 BSA. Before the assay, the αs proteins were incubated with 100 μM GTP[γS] for 30 min (αs-WT) or 1 min (αs-AVDT). (D) The effects of the indicated different guanine nucleotides on cAMP synthesis in the presence of 15 nM αs-WT or αs-AVDT. Reactions were conducted at 20°C for 10 min as described in C. Before the assay commenced, the αs proteins were incubated with GTP[γS], GTP, or guanosine 5′-[β-thio]diphosphate (GDP[βS]), each at 100 μM, or with 20 μM AlCl3 and 10 mM NaF for 30 min (αs-WT) or 1 min (αs-AVDT). (E) The protein stability of αs-WT or αs-AVDT assessed in vitro by [35S]GTP[γS] binding. Recombinant αs-WT or αs-AVDT (each at 30 nM) was incubated at 22°C with the indicated concentrations of GTP or GTP[γS]. At the times indicated, 5-μl aliquots were withdrawn and assayed for GTP[γS] binding in 20-μl reaction mixtures. The values represent the means ± SD of triplicate determinations, and each set of results is representative of at least two additional experiments.