Abstract
Background
The anterior‐medial thalamus (AMT), which is associated with memory processing, is severely affected by Alzheimer's disease pathology and, when damaged, can be the sole cause of dementia.
Objective
To assess the frequency of magnetic resonance imaging (MRI) hyperintensities affecting the AMT, and their relationship with sudden cognitive decline.
Methods
205 consecutive participants from a university cognitive neurology clinic underwent clinical evaluation, neuropsychological testing and quantitative MRI.
Results
AMT hyperintensities >5 mm3 occurred in 0 of 34 normal controls but were found in 5 of 30 (17%) participants with cognitive impairment with no dementia (CIND), 9 of 109 (8%) patients with probable Alzheimer's disease, 7 of 17 (41%) with mixed disease and 8 of 15 (53%) with probable vascular dementia (VaD). AMT hyperintensities occurred more often in participants with stepwise decline than in those with slow progression (χ2 = 31.7; p<0.001). Of the 29 people with AMT hyperintensities, those with slow progression had smaller medial temporal width (p<0.001) and smaller anterior‐medial thalamic hyperintensities (p<0.001). In a logistic regression model, both variables were significant, and the pattern of decline was correctly classified in 86% of the sample (Cox and Snell R2 = 0.56; p<0.001). Those with AMT hyperintensities >55 mm3 were likely to have stepwise decline in cognitive function regardless of medial temporal lobe width; in contrast, those with smaller AMT hyperintensities showed a stepwise decline only in the absence of medial temporal lobe atrophy. All patients with VaD had left‐sided AMT hyperintensities, whereas those with CIND had right‐sided AMT hyperintensities.
Conclusions
AMT hyperintensities >55 mm3 probably result in symptomatic decline, whereas smaller lesions may go unrecognised by clinicians and radiologists. Only half of those with AMT hyperintensities had diagnoses of VaD or mixed disease; the other AMT hyperintensities occurred in patients diagnosed with Alzheimer's disease or CIND. These silent hyperintensities may nevertheless contribute to cognitive dysfunction. AMT hyperintensities may represent a major and under‐recognised contributor to cognitive impairment.
Dementia caused solely by cerebrovascular disease is rare. In a large memory clinic autopsy series of over 1900 people with dementia, only six had infarcts without any Alzheimer's disease neuropathology,1 and all six people had infarctions affecting at least one of three key areas: the thalamus, the medial temporal lobe and the frontal cortex.1 Although the medial temporal lobe has long been appreciated as a site of strategic importance for dementia, the involvement of the thalamus is less frequently assessed. The anterior nucleus of the thalamus is part of a cortical network, including the hippocampus, anterior cingulate and mamillary bodies, which mediates memory processing.2,3,4,5 Infarcts to the anterior and dorsomedial thalamus are associated with memory impairment in animal studies,6 human case reports7,8,9,10 or series.1,11,12,13,14 One indication that thalamic infarcts may be important in dementia populations comes from the Nun Study, which found that people with infarcts to the basal ganglia, thalamus and deep white matter exhibited dementia with less Alzheimer's disease neuropathology than in those without infarcts.15
Despite this finding, and despite the appreciated role of anterior‐medial thalamic (AMT) infarcts in causing isolated cases of amnesia or dementia in stroke populations,11,12,13,16 the frequency and consequences of thalamic lesions in a large sample of people with cognitive impairment have not been evaluated. In this study, we quantified hyperintensities on magnetic resonance images (MRI) in the anterior‐medial thalamus in a cognitive neurology clinic sample. We determined the frequency and volumes of thalamic hyperintensities and whether these hyperintensities were associated with sudden changes in cognitive status defined by clinical history.
Materials and methods
Participants
Participants consisted of consecutive eligible patients from the Sunnybrook Cognitive Neurology Clinic, University of Toronto, Toronto, Canada, as well as community volunteers. All patients underwent a standard neurological examination and routine biochemical screening, and all participants underwent cognitive testing and MRI. Participants were excluded if the MRI and neuropsychological testing were separated by >10 weeks, or if magnetic resonance scans were technically inadequate for volumetric analysis. Participants had to be fluent in English to be included. Despite the more common incidence of vascular disease in older people, there are many case studies of young people with cognitive impairment after thalamic strokes. Therefore, young age was not an exclusion criterion. Participants ranged in age from 37 to 89 years. Normal controls were community‐dwelling people between 43 and 82 years old, who had to be free of both subjective and objective cognitive impairment. Possible secondary causes of dementia (other than vascular disease) and concomitant neurological or psychiatric illnesses were exclusionary for both controls and patients. In addition to clinical assessment, the Geriatric Depression Scale and a behavioural questionnaire (the Disability Assessment for Dementia), two commonly used screens in dementia populations, were applied to rule out concomitant exclusionary illnesses.
Dementia was defined using Diagnostic Statistical Manual for Mental Disorders—fourth edition criteria.17 Participants not meeting the criteria for dementia, but with subjective and objective impairment in cognitive function, were considered “cognitively impaired, no dementia” (CIND).18 This category therefore included participants with vascular cognitive impairment who did not meet the Diagnostic Manual for Mental Disorders criteria for dementia, as well as participants with amnestic‐type mild cognitive impairment or with multidomain, but mild, cognitive impairment. Those with dementia were diagnosed with probable vascular dementia (VaD) if they satisfied the NINDS‐AIREN criteria,19 and the diagnosis of probable Alzheimer's disease was made according to the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association criteria.20 Participants meeting the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association clinical criteria for Alzheimer's disease, but who also had vascular disease as a potential secondary contributing factor were considered to be possible candidates for Alzheimer's disease (with vascular disease, Alzheimer's disease and cardiovascular disease) according to the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association designations.20 The protocol was reviewed and approved by the institutional research ethics board, and written informed consent was obtained from all participants or their legal guardians.
Demographic information (table 1) was collected on all participants, including sex, age at time of magnetic resonance scan and duration of cognitive impairment. The Mini‐Mental State Examination21 was used to briefly assess global cognitive function. Information on the pattern of onset or progression of cognitive impairment was collected via patient and family histories. The pattern of cognitive decline in each participant was categorised as either slowly progressive or stepwise. To be considered a “stepwise” decline, a clear history of sudden onset or decline in functional and cognitive abilities, temporally related to other neurological deficits or to MRI evidence of strokes in the appropriate anatomical location, was required. Two types of stepwise decline were identified: (1) a sudden onset of cognitive decline, temporally related to a new stroke event (required for the diagnosis of VaD and also seen in patients with CIND from vascular causes) and (2) a sudden decline in cognitive or functional abilities in participants with pre‐existing cognitive decline (in patients with pre‐existing probable Alzheimer's disease, VaD or CIND in whom a new stroke occurred).
Table 1 Demographic information by diagnostic groups.
| NC (n = 34) | CIND (n = 30) | Probable AD (n = 109) | AD+CVD (n = 17) | Probable VaD (n = 15) | |
|---|---|---|---|---|---|
| Sex (female:male) | 20:14 | 13:17 | 54:55 | 9:8 | 6:9 |
| Age in years (minimum; maximum) | 67 (9) (43; 82) | 69 (9) (50; 88) | 72 (10) (37; 89) | 77 (6) (64; 89) | 78 (8) (59; 87) |
| Education in years | 15 (3) | 14 (3) | 14 (4) | 14 (3) | 14 (4) |
| MMSE | 29 (1) | 27 (2) | 22 (5) | 21 (4) | 22 (4) |
| Duration (years) | N/A | 2.8 (1.9) | 4.2 (2.9) | 4.7 (2.7) | 5.1 (5.6) |
AD, Alzheimer's disease; CIND, cognitively impaired, no dementia; CVD, cerebrovascular disease; MMSE, Mini‐Mental State Examination; N/A, not applicable; NC, normal control; VaD, vascular dementia.
Values are mean (SD) unless otherwise specified.
Magnetic resonance imaging
All brain images were acquired using a 1.5‐T Signa MR imager (GE Medical Systems, Milwaukee, Wisconsin, USA). A 12‐min, standard interleaved spin‐echo acquisition was carried out in the axial plane covering the whole brain, including the cerebellum. T2‐weighted and proton density‐weighted MRIs were acquired without gaps using 3‐mm‐thick slices (TE (echo time) = 30, 80 ms; TR (repetition time) = 3000 ms, 0.5 excitations, field of view 20×20 cm, matrix 256×192). In addition, a 10‐min, three‐dimensional, axially acquired T1‐weighted MR scan (TE/TR = 5/35 ms; flip angle = 35°; one excitation; voxel dimensions = 0.86×0.86×1.3; field of view = 20×20 cm; matrix 256×192) was used for analysis of the medial temporal region. Images were transferred to a Sun workstation (Sun Microsystems, Mountain View, California, USA) for post‐processing protocols.
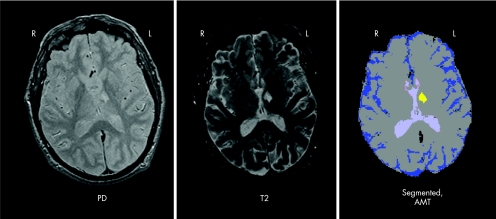
Volumetric brain measures were extracted from the T2‐proton density magnetic resonance images using a reliable protocol of segmentation followed by manual and automated post‐processing described previously.22 Briefly, using a standardised protocol, trained users selected “seed” training voxels representative of brain, cerebrospinal fluid and hyperintensities on interleaved T2‐proton density images. Using a k‐nearest neighbours algorithm, a fully segmented image was produced. Hyperintense voxels were further classified automatically, by direct connectivity to ventricles, as periventricular, whereas a user classified other hyperintense lesions as affecting the thalamus or basal ganglia on the basis of anatomical landmarks.22 Intraclass correlation coefficients for all variables were >0.95 for both inter‐rater and intra‐rater replication studies.22 Lesions within the anterior one third or medial one half of the thalamus were subclassified as AMT hyperintensities, and lesions in the lateral one half and posterior two thirds of the thalamus were classified as posterior‐lateral thalamic hyperintensities (see fig 1 for an example of a segmented image with the raw data in a person with a large anterior‐medial thalamic infarct). Lesions affecting the thalamus and the inferior genu of the internal capsule were considered as part of the AMT territory, as they affect both the anterior pole of the thalamus and disrupt the anterior thalamic peduncle, which conveys reciprocal connections between the dorsomedial nucleus and the cingulate gyrus, prefrontal and orbitofrontal cortices. Lesions disrupting these white matter pathways may have effects similar to lesions in the AMT.2 The intraclass correlation coefficients for both intra‐rater and inter‐rater reliability were >0.87 for all thalamic measures.
Figure 1 Images from a participant with VaD after a left‐sided anterior‐medial thalamic (AMT) infarct. Proton density‐weighted and T2‐weighted images of a single slice are shown, along with the corresponding slice from the segmented image.
The medial temporal lobe width (MTLW) was measured by a standardised protocol using individualised landmarks.23 Specifically, the slice corresponding to the intercollicular sulcus was chosen, with angulation oriented parallel to the long axis of the hippocampus, as described previously.23 The MTLW was measured at the thinnest point between the anterior and posterior borders of the mid‐brain.23 All measures were carried out in random order and blind to all demographic, clinical, neuropsychological and other quantitative imaging data.
Results
Frequency of AMT hyperintensities
Small hyperintensities (high signal on T2‐weighted and proton density‐weighted magnetic resonance images) can occur in deep grey matter structures owing to enlarged perivascular spaces or other non‐ischaemic changes. Thus, only AMT hyperintensities >5 mm3 (four voxels) were considered. AMT hyperintensities >5 mm3 did not occur in any of the 34 controls. In contrast, these hyperintensities were surprisingly common in our patient sample, affecting 29 of 171 (17%) people with cognitive impairment. Five of 30 (17%) participants with CIND had AMT hyperintensities. Of the 109 participants with probable Alzheimer's disease, 9 had AMT hyperintensities (8%). Seven of 17 (41%) participants with Alzheimer's disease and CVD had AMT hyperintensities. Finally, 8 of 15 (53%) participants who met the clinical criteria for probable VaD had AMT hyperintensities.
Relationship to pattern of cognitive decline
In the total sample of participants with cognitive impairment, AMT hyperintensities occurred significantly more often in participants with stepwise decline than in those with slow progression (Pearson's χ2 = 31.7; df = 1; p<0.001). Specifically, 14 of 25 (56%) people with stepwise decline had AMT hyperintensities, whereas only 15 of 146 (10%) of those with slow progression had these hyperintensities. The other 11 people with stepwise decline had large vessel strokes, affecting the medial temporal lobe, the parietotemporal association cortex or the posterior cingulate.
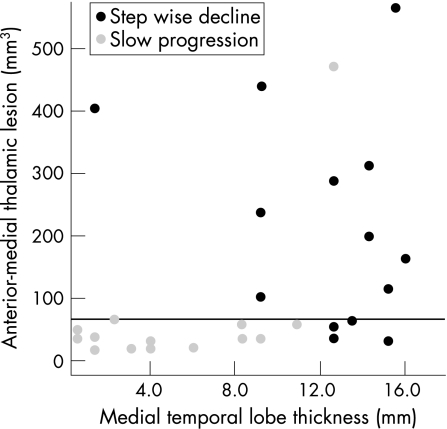
Within the group of 29 participants with AMT hyperintensities, there was a significant difference in both the medial temporal lobe width and the size of AMT hyperintensities between those with slow progression (n = 15) and those with a stepwise decline (n = 14) (fig 2). Specifically, those with slow progression had smaller medial temporal lobes (average width 5.3 v 12.5 mm in the stepwise group; t = −4.92; df = 27; two‐tailed p<0.001) and smaller AMT hyperintensities (intracranial capacity 0.004% v 0.015%; t = −4.08; df = 27; p<0.001). These differences were not affected by accounting for age and education in a multivariate general linear model. AMT and medial temporal lobe thickness were independent predictors of decline pattern, and correctly classified 86% of the sample (logistic regression, −2 log likelihood = 16.3; Cox and Snell R2 = 0.56; p<0.001). Age and number of years of education did not contribute to the model.
Figure 2 Cognitive decline and medial temporal lobe width for all participants with anterior‐medial thalamic hyperintensities ⩾5 mm3. Anterior‐medial thalamic hyperintensities >55 mm3 (horizontal line) resulted in a sudden onset or decline of cognitive function in 10 of 11 people. Smaller AMT hyperintensities (<55 mm3) in people with medial temporal atrophy (<12.0 mm) did not correlate with an acute change in cognitive function; in contrast, similarly small AMT hyperintensities (<55 mm3) in people without medial temporal atrophy did result in sudden decline (bottom right of graph).
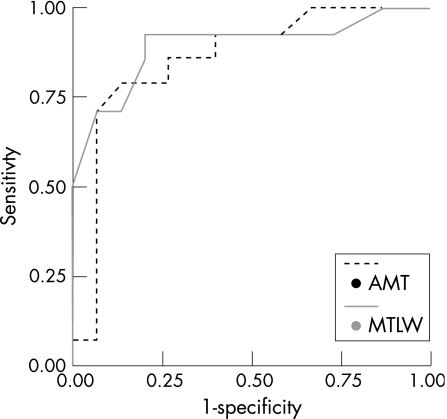
Cut‐off points for both AMT lesions and medial temporal lobe width were calculated for predicting pattern of decline (see supplemental data at http://jnnp.bmjjournals.com/supplemental). Participants with AMT hyperintensities >55 mm3 were very likely to show stepwise decline (fig 2). Intriguingly, several participants without medial temporal lobe atrophy showed a stepwise decline, even with smaller AMT hyperintensities (fig 2, bottom right). Figure 3 shows the receiver operating characteristic curves for both AMT hyperintensities and MTLW. Area under the curve for AMT was 0.86 and that for MTLW was 0.90.
Figure 3 Receiver operating characteristic curves for anterior‐medial thalamic (AMT) hyperintensities and medial temporal lobe width (MTLW) measures, for predicting a stepwise decline. Area under the curves: 0.90 (MTLW) and 0.86 (AMT).
Left–right differences among diagnostic groups
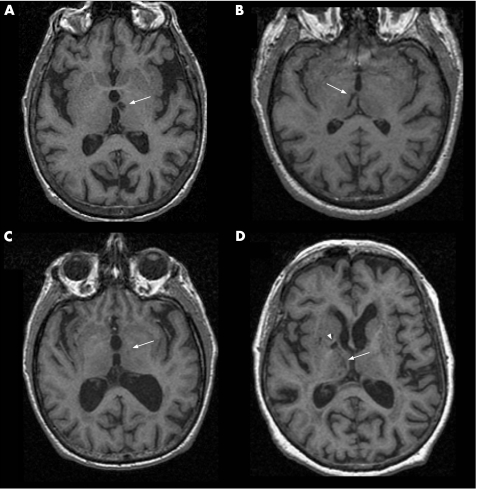
The effect of AMT hyperintensities differed by the side affected. Eight participants with VaD had AMT hyperintensities, and all were either bilateral or left‐sided (for a representative example, see fig 4A and table 2A). In contrast, the AMT hyperintensities were right‐sided in all five participants with CIND (one example shown in fig 4B and table 2B). Participants with probable Alzheimer's disease (one example shown in fig 4C and table 2C) or Alzheimer's disease and CVD (one example shown in fig 4D and table 2D) tended to have smaller hyperintensities with no consistent left or right localisation.
Figure 4 Typical thalamic hyperintensities in each diagnostic group.
Table 2 Demographics of the four participants in fig 4.
| Image | Dx | Age (years) | Sex | Duration (years) | MMSE | MTLW (mm) | AMT (mm3) | Stroke side |
|---|---|---|---|---|---|---|---|---|
| A | VaD | 84 | Female | 1.0 | 25 | 14.5 | 188 | Left |
| B | CIND | 71 | Male | 3.3 | 28 | 13.8 | 60 | Right |
| C | Probable AD | 77 | Male | 6.3 | 21 | 9.5 | 24 | Left |
| D | AD+CVD | 64 | Female | 2.3 | 27 | 12.9 | 461 | Right AMT and right MMT |
AD, Alzheimer's disease; AMT, anterior‐medial thalamic hyperintensity volume; CIND, cognitively impaired, no dementia; CVD, cerebrovascular disease; Dx, diagnosis; MMSE, Mini‐Mental State Examination; MMT, mamillothalamic tract (fig D, open arrowhead); MTLW, medial temporal lobe width; NC, normal control; VaD, vascular dementia.
Under‐recognition of AMT hyperintensities
Radiology reports often noted subcortical hyperintensities (called “non‐specific”, “age appropriate” or “in keeping with ischaemic vasculopathy”, depending on the severity). Thalamic hyperintensities were mentioned in only 8 of the 29 people with hyperintensities >5 mm3. In five of the eight people with VaD and AMT hyperintensities, the lesions were noted in the report. AMT hyperintensities were reported in only 1 of 16 people with clinical diagnoses of Alzheimer's disease or Alzheimer's disease with CVD. Of the five people with CIND who had AMT hyperintensities, only two were identified by report.
Discussion
AMT hyperintensities occurred more often than would be expected from the case report literature. They were found in roughly half of those with VaD or Alzheimer's disease and CVD, but were also seen in almost 10% of those with Alzheimer's disease and more than 15% of those with CIND. Indeed, 50% of AMT hyperintensities in this sample occurred in participants diagnosed with Alzheimer's disease or CIND, emphasising the heterogeneity of Alzheimer's disease. Previously, thalamic strokes have been cited as a cause of memory impairment in case reports and series.7,8,9,10,11,12,13,14 The frequency of these events has not been examined in a sample with dementia. One previous study investigated cognitive correlates of small thalamic lesions in a series of people with probable Alzheimer's disease. In that study, computed tomography scan hypodensities in the dorsomedial thalamus correlated with poorer attention and set shifting, in addition to learning and memory impairments.24 To our knowledge, the frequency of these lesions in controls, Alzheimer's disease, VaD, mixed and cognitive impaired populations has not been evaluated.
Infarct location is a key factor in determining both the severity and trajectory of cognitive impairment. Even the relatively subtle cognitive effects of white matter changes may be related to anatomical location25,26; so it is not surprising that a small number of limbic brain regions were identified in which vascular disease had sudden cognitive consequences. In this sample, the AMT region, along with the medial temporal lobe, the posterior cingulate and isocortical association areas, was affected in people with stepwise progression. These areas, and the white matter tracts that connect them (mamillothalamic tract and fornix), are commonly affected in published case studies of focal brain injury, causing a sudden onset of new cognitive impairment.2,3,4,5 Further, the same brain regions are affected early and severely by Alzheimer's disease neuropathology.27,28,29 It is thus not surprising that injury to such areas would emerge as the common cause of sudden decline in cognitive function in a tertiary referral clinic sample.
AMT hyperintensities were not always related to stepwise progression. In those participants with medial temporal lobe atrophy, it was only the larger AMT lesions (>55 mm3) that were associated with stepwise declines in function. However, in participants without medial temporal lobe atrophy, even smaller AMT lesions were related to stepwise declines in function. Thalamic lesions will probably have the largest effect in people with mild Alzheimer's disease pathology. One recent study has shown that thalamic and basal ganglia lacunes are related to impaired cognition in elderly people in the absence of amyloid plaques or Alzheimer's disease pathology.30 Clinicians and radiologists may often overlook these smaller lesions. In our observational series, AMT hyperintensities were frequently not mentioned in the radiologist's report. The larger, left‐sided hyperintensities in people with VaD were most likely to warrant comment. Without a clinical history of thalamic syndrome, and in the presence of global atrophy or small vessel disease, the thalamus was probably not scrutinised as a specific site of involvement; however, our results suggest that even smaller changes in the AMT may be more significant than previously appreciated.
It is possible that some of the smaller hyperintensities may be related to non‐vascular changes, such as Virchow–Robin spaces,31 and thus may have been under‐appreciated. However, Virchow–Robin spaces are typically not hyperintense on proton‐density images; further, as there were no hyperintensities >5 mm3 in the controls, these are probably not simply due to “innocent” ageing processes such as widening perivascular spaces. Additionally, a fluid‐attenuated inversion recovery magnetic resonance sequence is often used to aid differentiation between Virchow–Robin spaces and parenchymal lesions. However, T2‐weighted MRI is more sensitive than fluid‐attenuated inversion recovery for identifying thalamic lesions.32 If thalamic lesions are to be sought out and identified in ageing and dementia, caution must be used to avoid relying on fluid‐attenuated inversion recovery sequences. Diffusion‐weighted magnetic resonance sequences may be the most sensitive for detecting small thalamic lesions.33
Left‐sided thalamic lesions predominated in the vascular dementia sample, whereas right‐sided lesions were predominant in those with cognitive impairment not meeting criteria for dementia. This highlights the problem with operational definitions of dementia, which include memory and other cognitive domains. Left thalamic lesions are more likely to affect both verbal memory and language, whereas right‐sided lesions may result in more subtle memory deficits without language impairment, and thus may not “qualify” a person for a formal diagnosis of dementia. It is these patients who are expanding the conceptualisation of VaD towards a more inclusive consideration of “vascular cognitive impairment”. This terminology recognises the heterogeneous effects of vascular disease across a range of cognitive domains and severity.
Our results suggest that hyperintensities in the AMT >55 mm3 are likely to result in symptomatic decline. Smaller lesions may go unrecognised but may still be contributing to cognitive dysfunction, especially in those with minimal medial temporal lobe atrophy. Regardless of size, these structural brain changes are readily detected by standard clinical MRI, without requiring additional scanning sequences. In one large population study of “normal” people, even silent infarcts in the thalamus (ie, infarcts identified on MRI in people with no subjective cognitive complaints) were associated with a decline in memory performance.34 Elderly patients without plaques or tangles have impaired cognition with silent thalamic infarcts.30 Given that silent infarcts are more than five times as common as symptomatic infarcts,35,36 thalamic infarcts may represent a significant and under‐recognised contributor to cognitive impairment.
Acknowledgements
We thank Dr FQ Gao for assistance in collecting the medial temporal lobe measures, and Dr MJ Bronskill, Dr CB Caldwell, Dr P Stewart and Dr JP Szalai (now deceased) for their guidance. We also thank the ongoing dedication of the invaluable staff at the Cognitive Neurology group and the patients and their caregivers for their participation.
Abbreviations
AMT - anterior‐medial thalamus
CIND - cognitive impairment, no dementia
CVD - cerebrovascular disease
MRI - magnetic resonance imaging
MTLW - medial temporal lobe width
VaD - vascular dementia
Footnotes
Funding: This study was funded by the Canadian Institute of Health Research (CIHR) grant number MT13129 to SEB, and personal support was provided from CIHR with an MD/PhD studentship to RHS.
Competing interests: None declared.
References
- 1.Hulette C, Nochlin D, McKeel D.et al Clinical‐neuropathologic findings in multi‐infarct dementia: a report of six autopsied cases. Neurology 199748668–672. [DOI] [PubMed] [Google Scholar]
- 2.Tatemichi T K, Desmond D W, Prohovnik I. Strategic infarcts in vascular dementia. A clinical and brain imaging experience. Drug Res 199545371–385. [PubMed] [Google Scholar]
- 3.D'Esposito M, Verfaellie M, Alexander M P.et al Amnesia following traumatic bilateral fornix transection. Neurology 1995451546–1550. [DOI] [PubMed] [Google Scholar]
- 4.Duyckaerts C, Derouesne C, Signoret J L.et al Bilateral and limited amygdalohippocampal lesions causing a pure amnesic syndrome. Ann Neurol 198518314–319. [DOI] [PubMed] [Google Scholar]
- 5.Katayama K, Takahashi N, Ogawara K.et al Pure topographical disorientation due to right posterior cingulate lesion. Cortex 199935279–282. [DOI] [PubMed] [Google Scholar]
- 6.Aggleton J P, Neave N, Nagle S.et al A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behav Brain Res 19956891–101. [DOI] [PubMed] [Google Scholar]
- 7.Akiguchi I, Ino T, Nabatame H.et al Acute‐onset amnestic syndrome with localized infarct on the dominant side–comparison between anteromedial thalamic lesion and posterior cerebral artery territory lesion. Jpn J Med 19872615–20. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg G, Wimmer A, Maly J. Amnesic syndrome with a unilateral thalamic lesion: a case report. J Neurol 198322979–86. [DOI] [PubMed] [Google Scholar]
- 9.Squire L R, Moore R Y. Dorsal thalamic lesion in a noted case of human memory dysfunction. Ann Neurol 19796503–506. [DOI] [PubMed] [Google Scholar]
- 10.Mori E, Yamadori A, Mitani Y. Left thalamic infarction and disturbance of verbal memory: a clinicoanatomical study with a new method of computed tomographic stereotaxic lesion localization. Ann Neurol 198620671–676. [DOI] [PubMed] [Google Scholar]
- 11.Van der Werf Y D, Scheltens P, Lindeboom J.et al Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia 2003411330–1344. [DOI] [PubMed] [Google Scholar]
- 12.Graff‐Radford N R, Tranel D, Van Hoesen G W.et al Diencephalic amnesia. Brain 1990113(Pt 1)1–25. [DOI] [PubMed] [Google Scholar]
- 13.Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology 198838837–848. [DOI] [PubMed] [Google Scholar]
- 14.Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke 2004352826–2831. [DOI] [PubMed] [Google Scholar]
- 15.Snowdon D A, Greiner L H, Mortimer J A.et al Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997277813–817. [PubMed] [Google Scholar]
- 16.Castaigne P, Lhermitte F, Buge A.et al Paramedian thalamic and midbrain infarct: clinical and neuropathological study. Ann Neurol 198110127–148. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association, 1994
- 18.Graham J E, Rockwood K, Beattie B L.et al Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 19973491793–1796. [DOI] [PubMed] [Google Scholar]
- 19.Roman G C, Tatemichi T K, Erkinjuntti T.et al Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 199343250–260. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D A, Folstein M F.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Workgroup under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 21.Folstein M F, Folstein S E, McHugh P R. “Mini Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 22.Swartz R H, Black S E, Feinstein A.et al Utility of simultaneous brain, CSF and hyperintensity quantification in dementia. Psychiatry Res Neuroimaging 200211683–93. [DOI] [PubMed] [Google Scholar]
- 23.Gao F Q, Black S E, Leibovitch F S.et al A reliable MR measurement of medial temporal lobe width from the Sunnybrook Dementia Study. Neurobiol Aging 20032449–56. [DOI] [PubMed] [Google Scholar]
- 24.Forstl H, Sahakian B. Thalamic radiodensity and cognitive performance in mild and moderate dementia of the Alzheimer type. J Psychiatry Neurosci 19931833–37. [PMC free article] [PubMed] [Google Scholar]
- 25.Swartz R H, Sahlas D J, Black S E. Strategic involvement of cholinergic pathways and executive dysfunction: does location of white matter signal hyperintensities matter? J Stroke Cerebrovasc Dis 20031229–36. [DOI] [PubMed] [Google Scholar]
- 26.Bocti C, Swartz R H, Gao F Q.et al A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke 2005362126–2131. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 199182239–259. [DOI] [PubMed] [Google Scholar]
- 28.Braak H, Braak E. Staging of Alzheimer's disease‐related neurofibrillary changes. Neurobiol Aging 199516271–284. [DOI] [PubMed] [Google Scholar]
- 29.Brun A, Englund E. Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology 19815549–564. [DOI] [PubMed] [Google Scholar]
- 30.Gold G, Kovari E, Herrmann F R.et al Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke 2005361184–1188. [DOI] [PubMed] [Google Scholar]
- 31.Braffman B H, Zimmerman R A, Trojanowski J Q.et al Brain MR: pathologic correlation with gross and histopathology. 1. Lacunar infarction and Virchow‐Robin spaces. AJR Am J Roentgenol 1988151551–558. [DOI] [PubMed] [Google Scholar]
- 32.Bastos Leite A J, van Straaten E C, Scheltens P.et al Thalamic lesions in vascular dementia: low sensitivity of fluid‐attenuated inversion recovery (FLAIR) imaging. Stroke 200435415–419. [DOI] [PubMed] [Google Scholar]
- 33.Weidauer S, Nichtweiss M, Zanella F E.et al Assessment of paramedian thalamic infarcts: MR imaging, clinical features and prognosis. Eur Radiol 2004141615–1626. [DOI] [PubMed] [Google Scholar]
- 34.Vermeer S E, Prins N D, den Heijer T.et al Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 20033481215–1222. [DOI] [PubMed] [Google Scholar]
- 35.Bernick C, Kuller L, Dulberg C.et al Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology 2001571222–1229. [DOI] [PubMed] [Google Scholar]
- 36.Vermeer S E, Koudstaal P J, Oudkerk M.et al Prevalence and risk factors of silent brain infarcts in the population‐based Rotterdam Scan Study. Stroke 20023321–25. [DOI] [PubMed] [Google Scholar]