Abstract
Background
Retinal infarction and transient monocular blindness (TMB) are associated with an increased risk of future ischaemic stroke. Little information is available on the type of subsequent ischaemic strokes that may occur (anterior or posterior circulation and small vessel or large vessel).
Aim
To analyse the type of stroke after TMB.
Methods
Patients with transient or permanent retinal ischaemia were selected from three prospective studies: the Dutch TIA Trial, the Dutch Amaurosis Fugax Study and the European/Australian Stroke Prevention in Reversible Ischaemia Trial. On follow‐up the type of stroke was classified according to the supply territory and the type of vessel involved.
Results
654 patients were included. During a mean follow‐up of 5.2 years, 42 patients were found to have had a cerebral or retinal infarct, of which 27 occurred in the carotid territory ipsilateral to the symptomatic eye, 9 in the territory of the contralateral carotid artery and 6 were infratentorial strokes. Thirty patients had a large‐vessel infarct, four had a small‐vessel infarct and eight had a retinal infarct. Characteristics associated with a notable increased risk for subsequent stroke or retinal infarction were age ⩾65 years, a history of stroke, a history of intermittent claudication, diabetes mellitus, Rankin score ⩾3, more than three attacks of retinal ischaemia and any degree of ipsilateral carotid stenosis on duplex ultrasonography observation.
Conclusion
Ischaemic strokes after TMB or retinal infarction were found to be mainly large‐vessel infarcts in the territory of the ipsilateral carotid artery. TMB and retinal infarction are probably manifestations of large‐vessel disease.
Transient monocular blindness (TMB) is caused by temporary ischaemia of the retina in one eye, secondary to transient occlusion of a retinal artery, hypoperfusion or vasospasm.1 TMB is a risk factor for subsequent ischaemic stroke,2 but this risk is lower than after transient ischaemic attacks of the brain.3,4,5,6 The annual incidence of ischaemic stroke after TMB or retinal infarcts in unselected patients ranges between 2.0% and 2.8%,2,3,4,7,8,9 and increases up to 8.4% in the presence of high‐grade stenosis of the ipsilateral carotid artery.5 That TMB often occurs in patients with atherosclerotic lesions of the carotid artery3,6,10,11,12 suggests that it should be regarded as a manifestation of large‐vessel atherosclerosis. But, even among patients with stenosis of the internal carotid artery, the risk for subsequent stroke is lower in patients with TMB than in those who have had a cerebral transient ischaemic attack,5,6 which may indicate a difference in pathophysiological background between hemispheric and retinal ischaemia. Patients who have had a transient ischaemic attack or non‐disabling ischaemic stroke caused by large‐vessel disease are more likely to have subsequent large‐vessel stroke during follow‐up.13 If TMB is considered to be a manifestation of large‐vessel disease, the subsequent ischaemic stroke may be large‐vessel ischaemic stroke. To date, no information is available on whether brain infarcts after TMB are caused by small‐vessel or by large‐vessel disease, and whether subsequent ischaemic strokes occur mainly in the ipsilateral or also in the contralateral hemisphere, or in the vertebrobasilar territory. Given the low incidence of recurrent stroke after TMB, many patients have to be followed up for a long time for a meaningful analysis. We aimed to analyse the type of subsequent ischaemic stroke in patients with TMB or retinal infarction, to obtain more insight into the pathophysiology of retinal ischaemia, especially with respect to the type of vascular disease.
Methods
Patients
Patients with transient or permanent retinal ischaemia were selected from the following three studies: Dutch TIA Trial (DTT),14 Dutch Amaurosis Fugax Study (DAS)15 and the European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT).16,17
Between 1986 and 1989, the DTT included 3150 patients with transient or minor disabling cerebral or ocular ischaemia, of whom 2473 had an extended follow‐up assessment of >10 years in the Life Long After Cerebral Ischaemia Study (LILAC).18 Patients in the DTT were randomised between 38 and 283 mg of aspirin daily; about half of them were also randomised between 50 mg atenolol and placebo. Patients with cardioembolic attacks or defined causes other than arterial atherosclerosis were excluded.
The DAS (1993–6) included 341 patients with a sudden transient loss of vision in one eye in the previous 6 months, in whom an overt ophthalmological cause had been excluded. The patients were treated with antithrombotic drugs and, if necessary, with carotid endarterectomy, in accordance with the opinion of the doctor who was treating them. Patients with a cardioembolic cause were excluded from this study.
In the ESPRIT, which is an ongoing study, 2838 patients were randomised between 1997 and June 2004. Patients with transient or minor disabling cerebral or ocular ischaemia of presumed arterial origin were randomised to one of three different treatment regimens: aspirin 30–325 mg daily; aspirin 30–325 mg daily combined with dipyridamole 200 mg twice daily; or oral anticoagulation with target international randomised ratio values 2.0–3.0.
At study entry to all three trials, an ECG was requested for all patients. Brain imaging was not mandatory. Further cardiac investigation was carried out only if there was a clinical indication (eg, history of cardiac disease, palpitations).
Characteristics
Other than the usual biographical data and vascular risk factors, the following baseline characteristics were collected: Rankin score, duration of symptoms, number of attacks, side of retinal ischaemia and degree of stenosis of the ipsilateral internal carotid artery on duplex ultrasonography. Stroke and retinal infarction at baseline were diagnosed according to the same criteria as those used for the outcome events.
Outcome events
Information about the occurrence of ischaemic strokes and retinal infarcts during follow‐up was collected on follow‐up visits or through telephone interviews at intervals of 3 and 6 months, classified by an auditing committee and reassessed for this study. Ischaemic stroke was diagnosed when the neurological deficits had occurred suddenly and had persisted for more than 24 h. They had to correspond with a new infarct on CT examination or MRI, or had to cause an increase in handicap of at least one point on the modified Rankin scale.19 The strokes were classified according to the vascular territory in which they occurred: ipsilateral or contralateral carotid territory, or posterior circulation (subdivided into supratentorial and infratentorial). Ischaemic stroke during follow‐up was subdivided into large‐vessel and small‐vessel infarction. Large‐vessel infarction was diagnosed in the case of relevant infarct with a diameter > 15 mm on the CT scan or MRI, or in the case of a normal scan if the neurological deficit included the so‐called “cortical” signs, such as haemianopia, neglect or a language disorder. Large‐vessel infarcts were subdivided into cortical infarcts, large subcortical infarcts and borderzone infarcts. Small‐vessel infarction was diagnosed in case of a relevant subcortical or brainstem infarct with a diameter < 15 mm on the CT scan or MRI, or in the case of normal scan if the neurological deficit included one of the traditional lacunar syndromes.20 All CT scans and MRI (baseline and for outcome events) were assessed by three doctors before this study began. The diagnosis of retinal infarction was made if the loss of vision in one eye persisted or if funduscopic examination showed typical changes.
Data analyses
The duration of follow‐up was calculated in the DTT from randomisation to the time of close‐out visit, extended assessment (LILAC study), or the occurrence of death, stroke or retinal infarction; in the DAS from inclusion of the study until close‐out, death, stroke or retinal infarction; and in the ESPRIT from randomisation to the last visit, death, stroke or retinal infarction. Only first events were included in this study. To assess the relationship of each baseline characteristic with outcome, we carried out Cox's proportional hazards analyses. The cut‐off points of variables were defined in advance. The results are reported as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). If monocular blindness occurred on both sides (on separate occasions), subsequent events were counted as ipsilateral. Variables found to be related to the outcome on univariate analysis with p<0.10 were entered into a multivariate model, with a forward selection of variables.
Results
Baseline characteristics
In total, 654 patients with transient or permanent retinal ischaemia were included in this study: 174 patients from the DTT (149 of these had extended follow‐up in the LILAC study), 332 patients from the DAS (nine of these were excluded because of atrial fibrillation) and 148 patients from the ESPRIT. In 57 (8.8%) of the included patients, monocular blindness lasted for more than 24 h or loss of vision was permanent. Fifty seven per cent of the patients experienced more than one attack. Table 1 lists the baseline characteristics of the patients; their mean age was 63 years and 61% were men. The mean duration of follow‐up was 5.2 (SD 4.0) years. It was longest for patients from the DTT (9.5 years) because of the extended follow‐up in the LILAC study.
Table 1 Baseline characteristics of 654 patients with transient monocular blindness or retinal infarction.
| Characteristics | |
|---|---|
| Demographic characteristics | |
| Mean follow‐up in years (SD) | 5.2 (4.0) |
| Mean age in years (SD) | 62.7 (11.3) |
| Male | 399 (61.0) |
| Risk factors | |
| History of stroke | 54 (11.3) |
| History of angina pectoris | 125 (19.1) |
| History of myocardial infarction | 81 (12.4) |
| History of hypertension | 292 (44.6) |
| History of intermittent claudication | 87 (13.3) |
| History of hyperlipidaemia | 195 (29.8) |
| Diabetes mellitus | 37 (5.7) |
| Cigarette smoking | 266 (40.7) |
| Status on study entry | |
| Rankin score ⩾3 | 13 (2.0) |
| Systolic blood pressure ⩾160 mm Hg | 244 (37.3) |
| Diastolic blood pressure ⩾90 mm Hg | 284 (43.4) |
| Previous vascular intervention or operation | 109 (16.7) |
| Presentation | |
| Duration of attack >24 h | 57 (8.8) |
| Number of attacks >3 | 164 (25.3) |
| Left eye | 245 (51) |
| Both eyes (alternating) | 3 (0.6) |
| Duplex ultrasonography of ipsilateral internal carotid artery* | |
| Normal | 153 (46.1) |
| 1–69% stenosis | 33 (9.9) |
| 70–99% stenosis | 96 (28.9) |
| Occlusion | 45 (13.6) |
| Any stenosis | 174 (52.4) |
Values are n (%) unless otherwise stated.
*Data on the degree of carotid stenosis were available only in the Dutch Amaurosis Fugax Study.
Outcome events
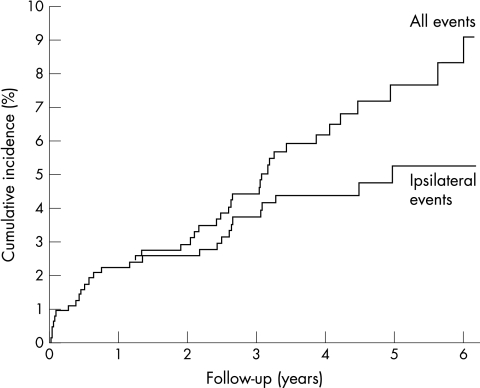
Forty two ischaemic strokes or retinal infarcts occurred during follow‐up. At 1 year and 5 years 586 and 182 patients, respectively, were still at risk. The cumulative 5‐year incidence was 7.7% (95% CI 5.2% to 10.2%). After the first year, the cumulative incidence was 2.2% (1.1% to 3.4%). Figure 1 shows a Kaplan–Meier curve of the cumulative incidence of ischaemic stroke and retinal infarction after TMB. The cumulative 5‐year incidence of ipsilateral carotid stroke or retinal infarction was 5.3% (3.2% to 7.3%; fig 1) and that of contralateral carotid or vertebrobasilar territory stroke or retinal infarction was 2.6% (1.0% to 4.1%). None of the patients had a stroke in the contralateral hemisphere or vertebrobasilar territory during the first year of follow‐up.
Figure 1 Cumulative incidence of ischaemic stroke and retinal infarction in 654 patients with transient monocular blindness or retinal infarction.
Table 2 shows the subtypes of the 42 outcome events after TMB (28 events occurred in the DAS).
Table 2 Stroke subtype and localisation after transient monocular blindness.
| Carotid territory | Total | |||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Infratentorial | ||
| Retinal infarct | 6 | 2 | 8 | |
| Large‐vessel infarct | 18 | 6 | 6 | 30 |
| Subcortical | 3 (3) | 1 (1) | 4 | |
| Cortical | 14 (10) | 4 (3) | 18 | |
| Borderzone | 1 (1) | 1 (1) | 2 | |
| Brain stem | 6 (0)* | 6 | ||
| Small‐vessel infarct | 3 | 1 | 4 | |
| Lacunar | 3(1) | 1 (1) | 4 | |
| Total | 27 | 9 | 6 | 42 |
Values in parentheses indicate the number of visible infarcts on CT scan or MRI of the brain.
*Five patients did not undergo CT scan or MRI of the brain.
Twenty seven ischaemic events occurred in the carotid territory ipsilateral to the TMB (all in the supply territory of the middle cerebral artery); nine occurred in the contralateral territory to the TMB. Six strokes occurred in the infratentorial region and were diagnosed from the clinical features by a normal CT scan (1) and without brain imaging (5). Four of these five patients had clinical signs of basilar artery thrombosis and subsequently died.
Ipsilateral carotid endarterectomy was carried out in 82 patients (all in the DAS study). Eight strokes occurred after carotid endarterectomy. One lacunar infarct, three cortical infarcts and one retinal infarct occurred in the territory of carotid surgery several years after endarterectomy.
A total of 30 large‐vessel infarcts occurred (4 subcortical, 18 cortical (territorial), 2 borderzone, 6 brain stem). Of these, 11 were classified on clinical grounds alone, because no relevant ischaemic lesions were observed on an early CT scan. Four patients had a lacunar syndrome; CT scanning showed a lacunar infarct in two of them. Eight patients had an ipsilateral retinal infarction during follow‐up. In all patients with a retinal infarct, a retinal artery occlusion was visible on funduscopy.
Three cortical territorial infarcts occurred in the perioperative period after carotid surgery (one contralateral to the side of TMB). Atrial fibrillation was found in four other patients with a subsequent large‐vessel infarct (one ipsilateral and two contralateral to the TMB and one basilar artery stroke). In 15 patients with an outcome event, no information was documented on the presence or absence of atrial fibrillation at the time of the event.
Characteristics of outcome
The CIs of the hazard ratios (HRs) based on data from the individual studies showed considerable overlap. Therefore, we considered pooling the data from the three studies to be appropriate. Table 3 shows the results of the univariate analyses for the risk of any subsequent ischaemic stroke or retinal infarction after TMB. Characteristics associated with a statistically significant increased risk for subsequent stroke or retinal infarction were age ⩾65 years, a history of stroke, a history of intermittent claudication, diabetes mellitus, Rankin score ⩾3, more than three attacks of retinal ischaemia and any degree of ipsilateral carotid stenosis on duplex ultrasonography. On multivariate analysis, diabetes was found to be the strongest predictor (HR 3.7; 95% CI 1.6 to 8.9), followed by the occurrence of more than three attacks (2.6; 1.4 to 4.8). On multivariate analyses with data from only the DAS, which included information on carotid stenosis, the strongest predictor remained diabetes with an HR of 5.2 (2.2 to 12.2), followed by any stenosis on the carotid duplex with an HR of 7.7 (2.3 to 25.6).
Table 3 Univariate analyses: stroke risk after transient monocular blindness.
| Characteristics | Any ischaemic stroke and retinal infarction (n = 42) | |
|---|---|---|
| HR | 95% CI | |
| Demographic characteristics | ||
| Male | 1.4 | 0.7 to 2.8 |
| Age ⩾65 years | 1.8 | 1.0 to 3.3 |
| Risk factors | ||
| History of stroke | 2.8 | 1.3 to 6.3 |
| History of angina pectoris | 1.0 | 0.5 to 2.2 |
| History of myocardial infarction | 1.6 | 0.7 to 3.6 |
| History of hypertension | 1.6 | 0.9 to 2.9 |
| History of intermittent claudication | 2.2 | 1.1 to 4.5 |
| History of hyperlipidaemia | 1.3 | 0.6 to 2.5 |
| Diabetes mellitus | 4.3 | 1.9 to 9.8 |
| Cigarette smoking | 1.7 | 0.9 to 3.0 |
| Status on study entry | ||
| Rankin score ⩾3 | 4.9 | 1.5 to 15.8 |
| Systolic blood pressure ⩾160 mm Hg | 1.1 | 0.6 to 2.1 |
| Diastolic blood pressure ⩾90 mm Hg | 1.1 | 0.6 to 2.1 |
| Previous vascular intervention or operation | 1.8 | 0.9 to 3.6 |
| Presentation | ||
| Duration of attack >24 h | 0.8 | 0.2 to 2.5 |
| Number of attacks >3 | 2.6 | 1.4 to 4.9 |
| Left eye | 1.1 | 0.5 to 2.2 |
| Duplex ultrasonography of ipsilateral internal carotid artery | ||
| Normal | reference | |
| 1–69% stenosis | 8.4 | 2.0 to 35.3 |
| 70–99% stenosis | 7.6 | 2.2 to 26.7 |
| Occlusion | 9.7 | 2.5 to 37.6 |
| Any stenosis | 8.5 | 2.6 to 28.2 |
HR, hazard ratio
Discussion
This study shows that brain infarcts after TMB or retinal infarction of presumed arterial origin are mainly infarcts in the supply territory of a large intracranial artery. These findings suggest that TMB and retinal infarction of presumed arterial origin should in general be considered to be manifestations of large‐artery atherosclerotic disease.
It is known that the risk of subsequent stroke is much smaller after TMB than after a cerebral transient ischaemic attack.2,3,4,5,6,21 Atrial fibrillation is more common with events in the brain than in the eye and, conversely, severe ipsilateral carotid disease is less common in the brain than in the eye.10,11 Moreover, a definite relationship has been reported between the degree of stenosis of the carotid artery and the risk of stroke after TMB.2,5,6 One possible explanation for a pathophysiological difference between cerebral and retinal ischaemia is that the smaller particles of the carotid atheromatous plaques can occlude the retinal artery, whereas larger emboli from the heart are too big to enter the retinal artery and are therefore more likely to occlude the cerebral arteries.10,11 Other possible explanations are that small particles are less likely to cause symptoms in the brain than in the eye, or that the cause of TMB is situated in the ophthalmic artery itself. The risk of subsequent stroke after retinal infarction is not known, but the pathophysiological mechanism and risk profile are probably similar.
If we consider TMB to be a manifestation of large‐vessel disease, why is its prognosis better than that of hemispheric episodes of ischaemia attributed to large‐vessel disease? About half of the patients in the DAS had a normal ipsilateral carotid artery on duplex ultrasonography. In these patients, the cause of the symptoms may not be atherosclerosis, but some other condition less likely to be followed by complications—for example, vasospasm.22,23 Furthermore, patients with transient ischaemia of the brain often have a cardioembolic source with a higher risk of stroke than those with large‐vessel atherosclerosis, the probable cause of TMB.24
The absolute risk for ipsilateral stroke after TMB is low, in this and other studies,2,3,4,7,8,9 but increases in patients with an ipsilateral carotid stenosis.5 Pooled data from the European Carotid Surgery Trial and the North American Symptomatic Carotid Endarterectomy Trial indicate that in patients with ocular ischaemia and with more than 70% stenosis of the internal carotid artery, carotid endarterectomy is probably effective.12
To the best of our knowledge, this study represents the largest group of patients with TMB or retinal infarction prospectively followed and described to date. It is also the first study with information about the subtype and vascular territory of subsequent ischaemic stroke in patients without cardioembolism. Our study, however, has some limitations. The first is that we combined the data from three different cohorts. The DTT was performed several years before the other two studies, which implies that in patients from the DTT, therapeutic intervention for some risk factors, especially hypertension, hyperlipidaemia and diabetes, was probably less intense than in those from the DAS or ESPRIT. For this reason, these risk factors were not analysed further. Theoretically, it is possible that the preventive treatment regimens had a differential effect on the risk of new vascular events in either large or small vessels, but our data do not permit verification of this assumption. Our study may not be perfect with regard to similarity of recruitment of the patients in the three studies. In our view, however, this is less of an issue, as we aimed only to analyse the type of stroke on follow‐up rather than assess the absolute frequency in a well‐defined group of patients. Also, most outcome events occurred in the DAS, in which patients for the observational study were not randomised. Furthermore, a rather high proportion (14%) of the patients in this study had an occlusion of the carotid artery and some patients had carotid endarterectomy during follow‐up. Carotid surgery may influence the natural history of monocular blindness and therefore this study is less suitable to estimate the absolute incidence rates. All patients were treated according to the standards of best care. This means that patients with severe stenosis of the internal carotid artery were advised to undergo carotid endarterectomy. If monocular blindness is regarded as a large‐vessel disease, it may appear, despite carotid endarterectomy, in other large vessels or even in the ipsilateral carotid territory at a later stage. Although we do not have complete information about all our patients, we assume that none of the patients in the ESPRIT had severe carotid stenosis at the beginning of follow‐up; no patient underwent carotid surgery; and patients who needed surgery were not included in the ESPRIT. The DTT was largely conducted before the period in which carotid surgery became a regular treatment for patients with high‐grade stenosis. Of the 30 strokes on follow‐up, no relevant infarcts were visible in eight and no scanning was carried out in five. Hence, classification of these events was based on the clinical features alone. This is probably only a minor problem, as only the method we used to distinguish large‐vessel strokes from small‐vessel strokes by means of clinical features has proved to be reliable in an earlier study.20 Finally, a cardiac source of embolism could not be ruled out in 15 patients. Nevertheless, we consider the preponderance of large‐vessel stroke on follow‐up to be so clear that these issues do not invalidate our main conclusion. Furthermore, we chose to use a classification method free from risk factors to distinguish small‐vessel infarcts from large‐vessel infarcts, and subsequently to obtain HRs without bias, as vascular risk factors are associated with each other.25
The conclusion that patients with retinal ischaemia are particularly at risk for subsequent large‐vessel infarcts may be useful in studies that compare large‐vessel and small‐vessel disease.
Acknowledgements
We thank the investigators of the DTIT, the DAS and the ESPRIT for making their data available for this study.
Abbreviations
DAS - Dutch Amaurosis Fugax Study
DTT - Dutch TIA Trial
ESPRIT - European/Australian Stroke Prevention in Reversible Ischaemia Trial
LILAC - Life Long After Cerebral Ischaemia Study
TMB - transient monocular blindness
Footnotes
Funding: All three studies were funded in part by The Netherlands Heart Foundation.
Competing interests: None declared.
References
- 1.Fisher M. Transient monocular blindness associated with hemiplegia. Am Arch Opthalmol 195247167–203. [DOI] [PubMed] [Google Scholar]
- 2.Poole C J, Ross Russell R W. Mortality and stroke after amaurosis fugax. J Neurol Neurosurg Psychiatry 198548902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurwitz B J, Heyman A, Wilkinson W E.et al Comparison of amaurosis fugax and transient cerebral ischemia: a prospective clinical and arteriographic study. Ann Neurol 198518698–704. [DOI] [PubMed] [Google Scholar]
- 4.Wilterdink J L, Easton J D. Vascular event rates in patients with atherosclerotic cerebrovascular disease. Arch Neurol 199249857–863. [DOI] [PubMed] [Google Scholar]
- 5.Streifler J Y, Eliasziw M, Benavente O R.et al The risk of stroke in patients with first‐ever retinal vs hemispheric transient ischemic attacks and high‐grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Arch Neurol 199552246–249. [DOI] [PubMed] [Google Scholar]
- 6.Benavente O, Eliasziw M, Streifler J Y.et al Prognosis after transient monocular blindness associated with carotid‐artery stenosis. N Engl J Med 20013451084–1090. [DOI] [PubMed] [Google Scholar]
- 7.Hankey G J, Slattery J M, Warlow C P. The prognosis of hospital‐referred transient ischaemic attacks. J Neurol Neurosurg Psychiatry 199154793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donders R C, Kappelle L J, Algra A.et al Subtypes of transient monocular blindness and subsequent risk of vascular complications. Cerebrovasc Dis 19966241–247. [Google Scholar]
- 9.Hankey G J, Slattery J M, Warlow C P. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ 1991302499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mead G E, Lewis S C, Wardlaw J M.et al Comparison of risk factors in patients with transient and prolonged eye and brain ischemic syndromes. Stroke 2002332383–2390. [DOI] [PubMed] [Google Scholar]
- 11.Anderson D C, Kappelle L J, Eliasziw M.et al Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke 2002331963–1967. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell P M, Eliasziw M, Gutnikov S A.et al Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004363915–924. [DOI] [PubMed] [Google Scholar]
- 13.Kappelle L J, van Latum J C, van Swieten J C.et al Recurrent stroke after transient ischaemic attack or minor ischaemic stroke: does the distinction between small and large vessel disease remain true to type? Dutch TIA Trial Study Group. J Neurol Neurosurg Psychiatry 199559127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Dutch TIA Trial Study Group A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med 19913251261–1266. [DOI] [PubMed] [Google Scholar]
- 15.Donders R C. Clinical features of transient monocular blindness and the likelihood of atherosclerotic lesions of the internal carotid artery. J Neurol Neurosurg Psychiatry 200171247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Schryver E L L M, on behalf of the European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) Group Design of ESPRIT: an international randomized trial for secondary prevention after non‐disabling cerebral ischaemia of arterial origin. Cerebrovasc Dis 200010147–150. [DOI] [PubMed] [Google Scholar]
- 17.De Schryver E L L M, on behalf of the European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) Group ESPRIT: protocol change. Cerebrovasc Dis 200111286 [Google Scholar]
- 18.van Wijk I, Kappelle L J, van Gijn J.et al Long‐term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet 20053652098–2104. [DOI] [PubMed] [Google Scholar]
- 19.Bamford J M, Sandercock P A G, Warlow C P.et al Interobserver agreement for the assessment of handicap in stroke patients [letter]. Stroke 198920828. [DOI] [PubMed] [Google Scholar]
- 20.Kappelle L J, van Latum J C, Koudstaal P J.et al Transient ischaemic attacks and small‐vessel disease. Dutch TIA Study Group. Lancet 1991337339–341. [DOI] [PubMed] [Google Scholar]
- 21.Anon Predictors of major vascular events in patients with a transient ischemic attack or nondisabling stroke. The Dutch TIA Trial Study Group. Stroke 199324527–531. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin J A, Gorelick P B, Helgason C M. Symptoms of amaurosis fugax in atherosclerotic carotid artery disease. Neurology 198737829–832. [DOI] [PubMed] [Google Scholar]
- 23.Donders R C J M.Transient monocular blindness. Diagnosis and prognosis of different subtypes [dissertation] The Netherlands, University Utrecht, 199957–74.
- 24.Kolominsky‐Rabas P L, Weber M, Gefeller O.et al Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long‐term survival in ischemic stroke subtypes: a population‐based study. Stroke 2001322735–2740. [DOI] [PubMed] [Google Scholar]
- 25.Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke 200536891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]