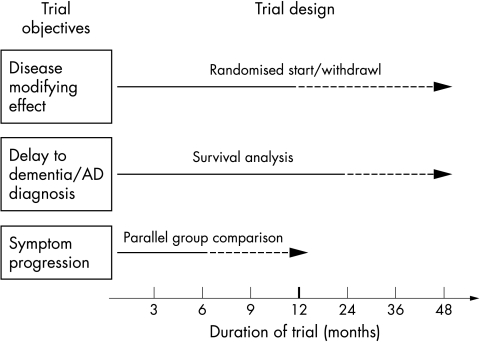
Figure 1 Primary objectives, theoretical designs, and optimal duration of clinical trials in MCI. Solid lines indicate minimum trial duration and dashed lines optimal trial duration. Probable disease modifying effect requires a randomised start or randomised withdrawal design,54,56 where after delayed start or withdrawal of active treatment the placebo group does not converge with the treatment group. The last design is a theoretical and has not been applied in either AD or MCI trials.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.