Abstract
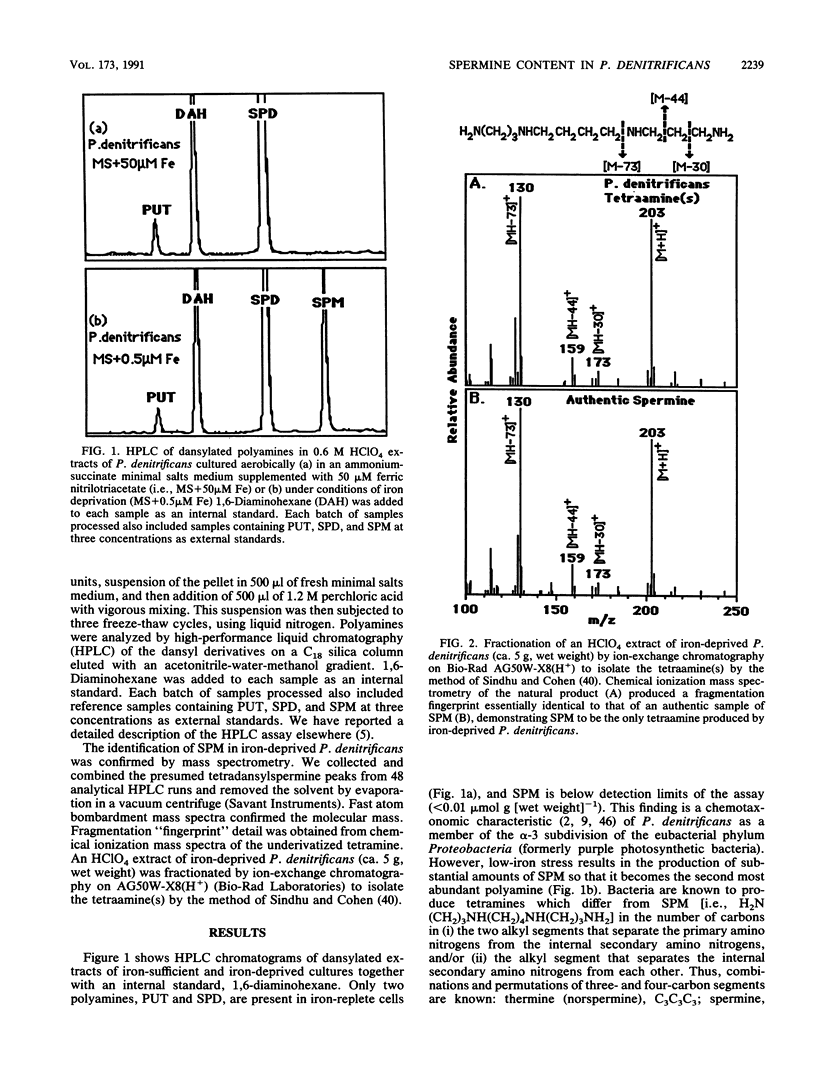
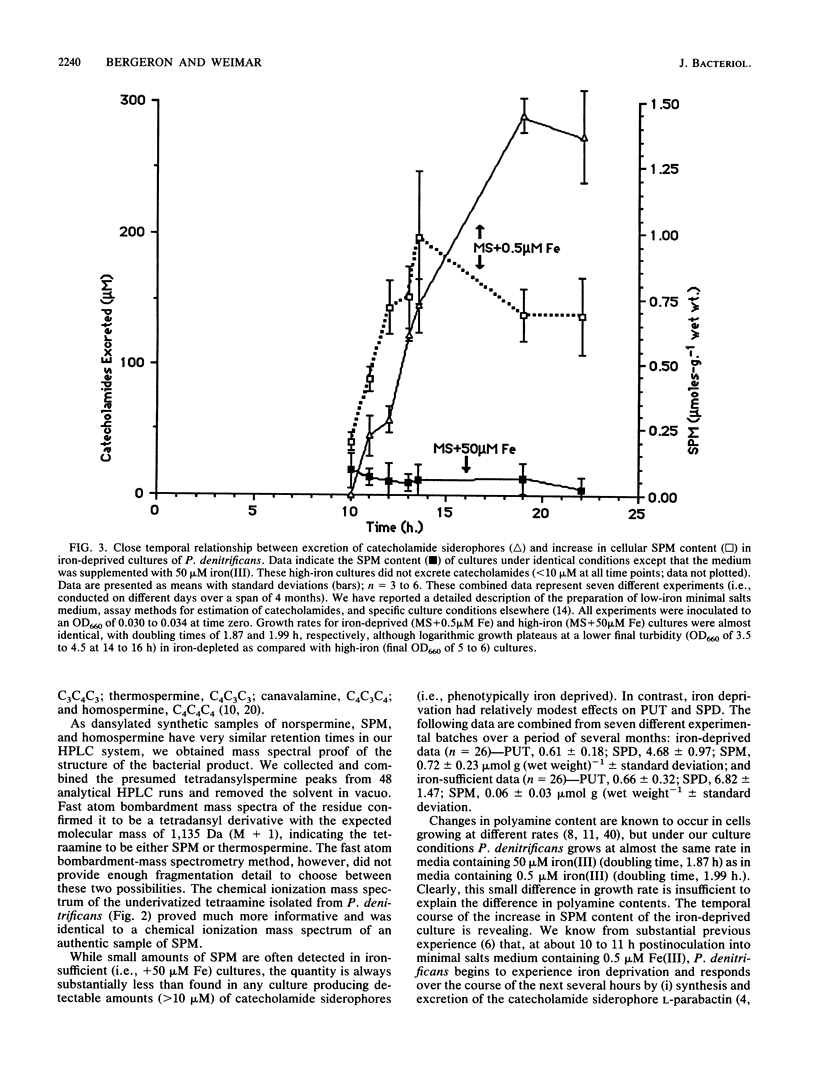
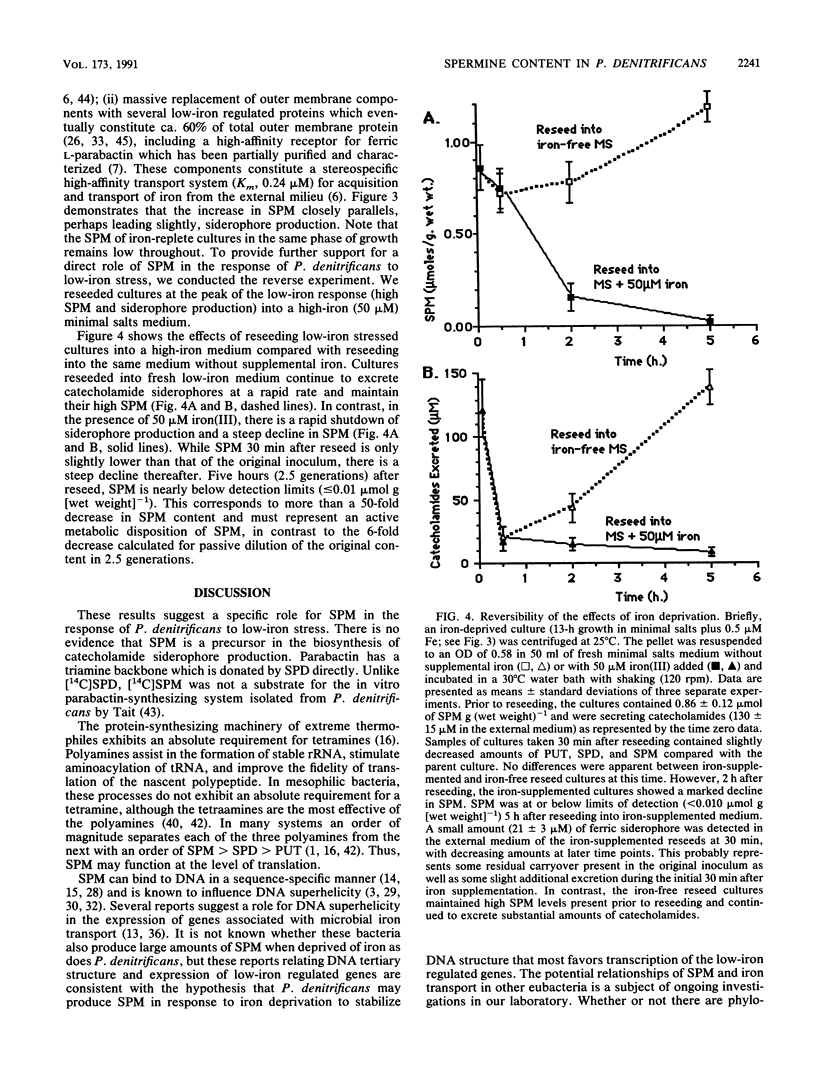
Spermine is present in relatively low amounts in Paracoccus denitrificans cultured aerobically in an ammonium succinate minimal salts medium supplemented with 50 microM iron(III). However, in iron-deprived cultures [minimal salts medium containing 0.5 microM iron(III)], spermine content increases by an order of magnitude in coordination with the well-known responses to iron derivation, e.g., derepression of siderophore synthesis and siderophore excretion. When iron-deprived cultures exhibiting both high spermine content and strong siderophore production are reseeded into fresh minimal salts medium containing 50 microM iron[III], both siderophore production and spermine content fall rapidly. Five hours after iron supplementation, spermine is below limits of detection. These results suggest a specific role for spermine in the response of P. denitrificans to low-iron stress.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu H. S., Feuerstein B. G., Deen D. F., Lubich W. P., Bergeron R. J., Samejima K., Marton L. J. Correlation between the effects of polyamine analogues on DNA conformation and cell growth. Cancer Res. 1989 Oct 15;49(20):5591–5597. [PubMed] [Google Scholar]
- Bergeron R. J., Dionis J. B., Elliott G. T., Kline S. J. Mechanism and stereospecificity of the parabactin-mediated iron-transport system in Paracoccus denitrificans. J Biol Chem. 1985 Jul 5;260(13):7936–7944. [PubMed] [Google Scholar]
- Bergeron R. J., Hawthorne T. R., Vinson J. R., Beck D. E., Jr, Ingeno M. J. Role of the methylene backbone in the antiproliferative activity of polyamine analogues on L1210 cells. Cancer Res. 1989 Jun 1;49(11):2959–2964. [PubMed] [Google Scholar]
- Bergeron R. J., Weimar W. R., Dionis J. B. Demonstration of ferric L-parabactin-binding activity in the outer membrane of Paracoccus denitrificans. J Bacteriol. 1988 Aug;170(8):3711–3717. doi: 10.1128/jb.170.8.3711-3717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., McCann P. P., Sjoerdsma A. Restriction of bacterial growth by inhibition of polyamine biosynthesis by using monofluoromethylornithine, difluoromethylarginine and dicyclohexylammonium sulphate. Biochem J. 1982 Nov 15;208(2):435–441. doi: 10.1042/bj2080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S. Some roles of polyamines in microbial physiology. Adv Enzyme Regul. 1972;10:207–223. doi: 10.1016/0065-2571(72)90015-5. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Barr G. C., Ni Bhriain N., Higgins C. F. DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol. 1988 Jun;170(6):2816–2826. doi: 10.1128/jb.170.6.2816-2826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Molecular dynamics of spermine-DNA interactions: sequence specificity and DNA bending for a simple ligand. Nucleic Acids Res. 1989 Sep 12;17(17):6883–6892. doi: 10.1093/nar/17.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara S., Harada Y. Fast-growing root nodule bacteria produce a novel polyamine, aminobutylhomospermidine. Biochem Biophys Res Commun. 1989 Dec 15;165(2):659–666. doi: 10.1016/s0006-291x(89)80016-6. [DOI] [PubMed] [Google Scholar]
- Hamana K., Akiba T., Uchino F., Matsuzaki S. Distribution of spermine in bacilli and lactic acid bacteria. Can J Microbiol. 1989 Apr;35(4):450–455. doi: 10.1139/m89-069. [DOI] [PubMed] [Google Scholar]
- Hamana K., Kamekura M., Onishi H., Akazawa T., Matsuzaki S. Polyamines in photosynthetic eubacteria and extreme-halophilic archaebacteria. J Biochem. 1985 Jun;97(6):1653–1658. doi: 10.1093/oxfordjournals.jbchem.a135223. [DOI] [PubMed] [Google Scholar]
- Majumder K., Brahmachari S. K. Sequence specificity in spermine-induced structural changes in CG-oligomers. Biochem Int. 1989 Feb;18(2):455–465. [PubMed] [Google Scholar]
- Marquet R., Houssier C. Different binding modes of spermine to A-T and G-C base pairs modulate the bending and stiffening of the DNA double helix. J Biomol Struct Dyn. 1988 Oct;6(2):235–246. doi: 10.1080/07391102.1988.10507710. [DOI] [PubMed] [Google Scholar]
- Marquet R., Wyart A., Houssier C. Influence of DNA length on spermine-induced condensation. Importance of the bending and stiffening of DNA. Biochim Biophys Acta. 1987 Aug 25;909(3):165–172. doi: 10.1016/0167-4781(87)90074-1. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Page W. J., Patrick J. The DNA gyrase inhibitors, nalidixic acid and oxolinic acid, prevent iron-mediated repression of catechol siderophore synthesis in Azotobacter vinelandii. Biol Met. 1988;1(1):57–61. doi: 10.1007/BF01128018. [DOI] [PubMed] [Google Scholar]
- Rosenberg H. Transport of iron into bacterial cells. Methods Enzymol. 1979;56:388–394. doi: 10.1016/0076-6879(79)56036-4. [DOI] [PubMed] [Google Scholar]
- Scalabrino G., Ferioli M. E. Polyamines in mammalian tumors. Part I. Adv Cancer Res. 1981;35:151–268. doi: 10.1016/s0065-230x(08)60911-2. [DOI] [PubMed] [Google Scholar]
- Scherer P., Kneifel H. Distribution of polyamines in methanogenic bacteria. J Bacteriol. 1983 Jun;154(3):1315–1322. doi: 10.1128/jb.154.3.1315-1322.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tait G. H. The identification and biosynthesis of siderochromes formed by Micrococcus denitrificans. Biochem J. 1975 Jan;146(1):191–204. doi: 10.1042/bj1460191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Weisburg W. G., Paster B. J., Madigan M. T., Fowler V. J., Hahn C. M., Blanz P., Gupta R., Nealson K. H. The phylogeny of purple bacteria: the alpha subdivision. Syst Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]


