Figure 1.
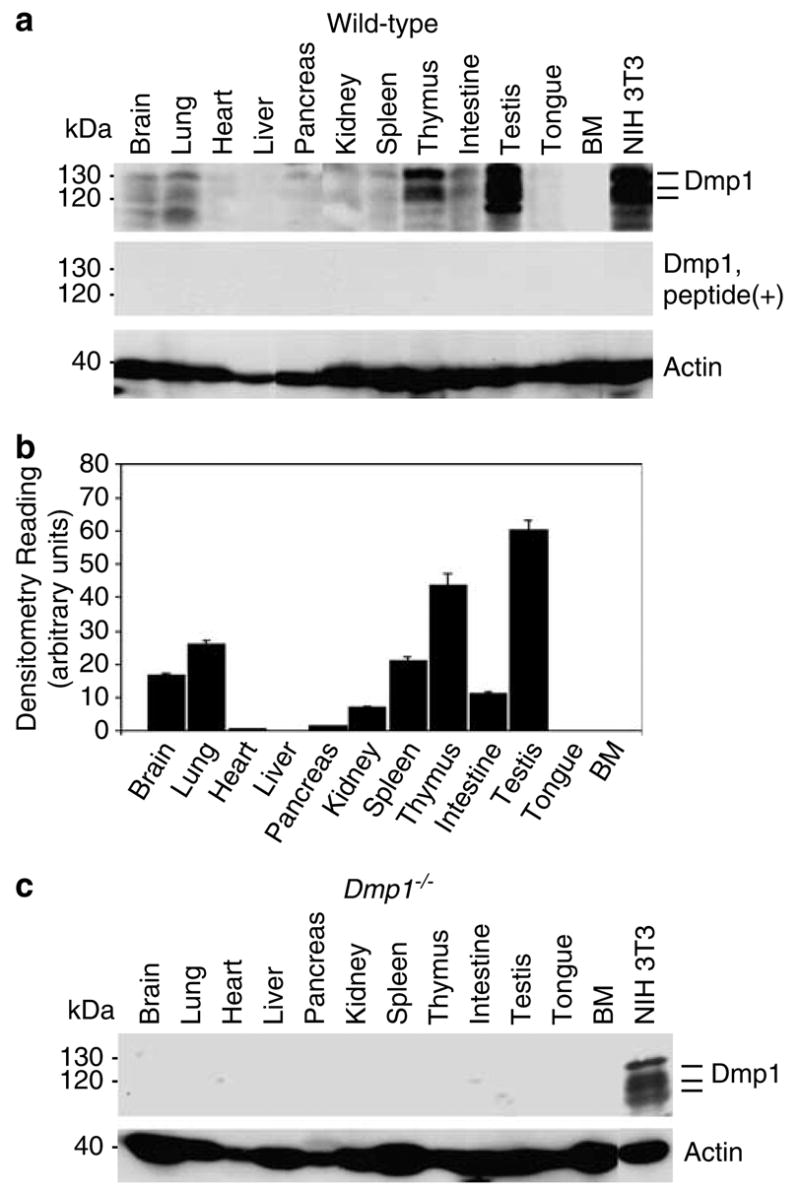
Distribution of the Dmp1 protein in normal murine tissues and the specificity of the anti-Dmp1 antibodies for immunohistochemistry. (a) Detection of the Dmp1 protein in an 8-week-old mouse using tissue samples with RAX antibodies. Indicator bars at right show the positions of multiple Dmp1 isoforms. Lysate from NIH 3T3 cells was used as a positive control. Equal protein loading was verified by blotting the filter with antibodies to actin. Top panel, Western blotting with RAX without antigenic peptide; middle panel, Western blotting with RAX preincubated with the antigenic peptide. (b) Histogram of densitometry of the immunoblots shown in A. The highest level of expression can be found in the testis, thymus, and lung. The Dmp1 protein was barely detectable in the heart, liver, pancreas, tongue, and bone marrow. The intensity of signals was calculated by using densitometric readings, and bars show mean optical density normalized to the control samples for each group ± s.e.m., n = 2, *P<0.05. (c) The Dmp1 protein is not detectable with RAX in tissues from an 8-week-old Dmp1−/− mouse.
