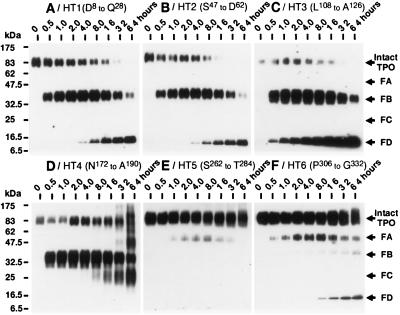
Figure 3.
The transition of domains in thrombin-cleaved components derived from intact rhTPO. Aliquots of thrombin-digested rhTPO, obtained at the same time intervals as described in the legend to Fig. 2, were further analyzed on immunoblots probed with various anti-TPO peptide Abs. Since the sensitivity to the antigen varied among the anti-TPO peptide Abs, appropriate amounts of the aliquots were applied onto SDS/PAGE gels to achieve maximal detection of each antigen. After incubation at 37°C for the times indicated, TPO peptide fragments on each immunoblot were visualized by the ECL method. These Western blots were proved by anti-HT1 Ab, 1 ng of rhTPO per lane (A); anti-HT2 Ab, 5 ng of rhTPO per lane (B); anti HT3 Ab, 100 ng of rhTPO per lane (C); anti-HT4 Ab, 50 ng of rhTPO per lane (D); anti-HT5 Ab, 500 ng of rhTPO per lane (E); anti-HT6 Ab, 500 ng of rhTPO per lane (F). The peptide domains recognized by the anti-TPO peptide Abs here are shown in Fig. 5.