Figure 5.
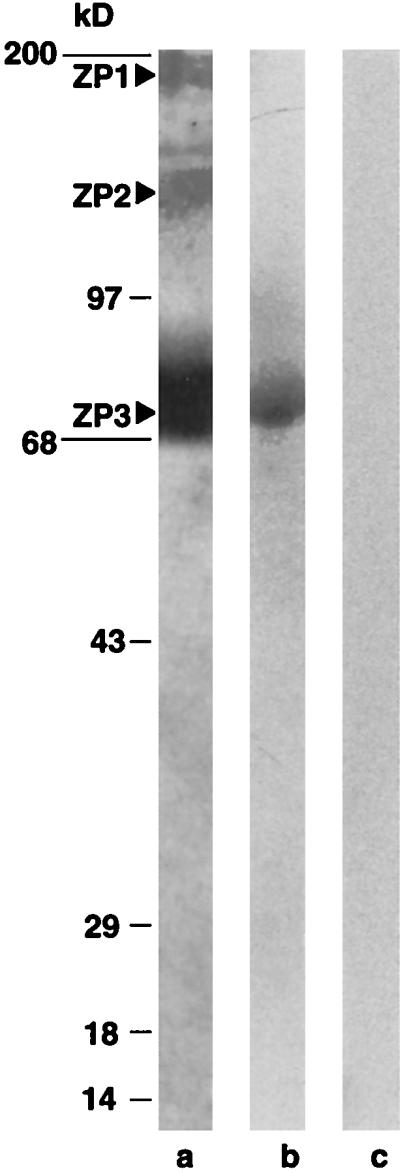
Interaction between the recombinant FA-1 protein and ZP3 of oocyte zona pellucida. The solubilized-zona pellucida preparation from murine oocytes was separated in SDS/PAGE (5–15%), and then transferred to nitrocellulose membrane for Western blot procedure. The recombinant protein was biotinylated and separated from unbiotinylated protein following the manufacturer’s protocol. The Western blot was incubated with biotinylated protein (≈1.5 μg/ml), washed, allowed to react with streptavidin-horseradish peroxidase (1:1,500), washed again, incubated with substrate, and exposed to x-ray film for 1–5 min. The solubilized zona pellucida preparation showed three bands (ZP1, ZP2, and ZP3, respectively) in SDS/PAGE (lane a). The recombinant FA-1 protein specifically recognized ZP3 on the Western blot (lane b). The biotinylated BSA used as a control did not react with any band on the Western blot (lane c).
