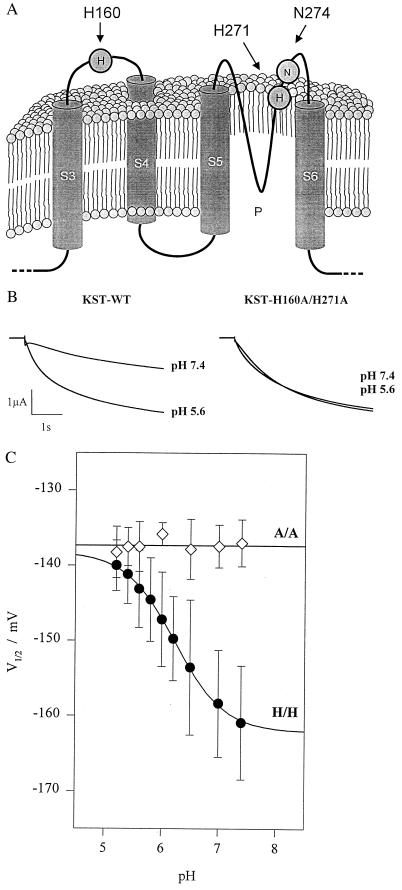
Figure 2.
pH-dependence of wild-type KST1 and the histidine double mutant. (A) Cartoon of the predicted topology of segments S3 to S6, including the pore region P and the extracellular linker between S3 and S4 [in relation to the Shaker K+ channel structure (18)]. (B) Compared with wild-type (left traces) inward currents of the double mutant KST1-H160A/H271A were not affected by changes in the extracellular proton concentration (right traces). (C) In contrast to the wild-type (H/H) substitution of both histidines by alanine (A/A) resulted in a loss of pH-sensitivity of the half-maximum activation potential. Data points represent the mean ± SE of three to six measurements, and solid lines represents best fits according to Eq. 2.