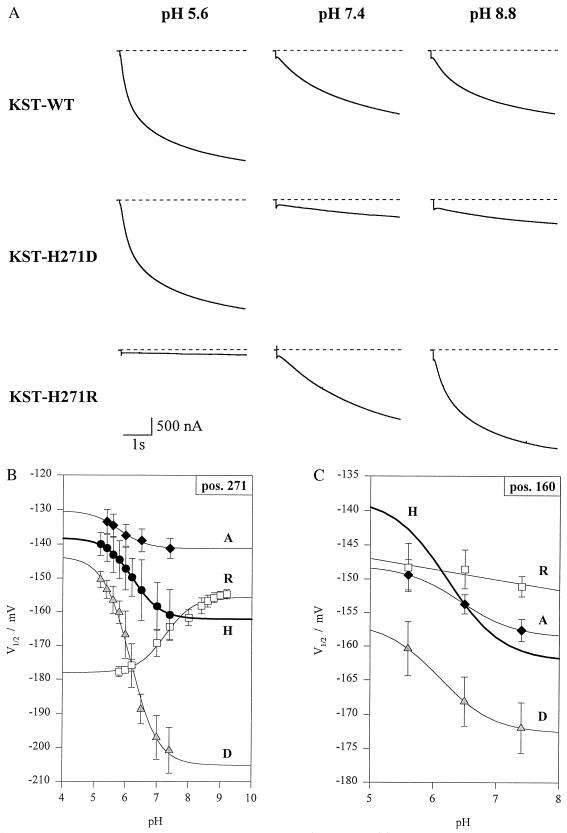
Figure 3.
pH-sensitivity of wild-type KST1 and single mutants. (A Top) Wild-type inward currents in response to pH 5.6, 7.4, and 8.8 in the bath medium. (Middle) Pronounced reduction of K+ current in mutant KST1–H271D in response to an increase in extracellular pH from 5.6 to 7.4 and 8.8 with respect to wild-type KST1 and KST1–H271R. (Bottom) The mutant KST1–H271R converts the acid activation into an alkaline activation. Note the absence of inward K+ current at pH 5.6 compared with wild-type KST1 and KST1–H271D. (B) pH-sensitivity of the half-activation potential (V½) in wild-type KST1 (H) and the mutants KST1–H271R (R), –H271D (D), and –H271A (A) (n = 3–6). (C) Reduction in pH dependence of the mutants KST1–H160R (R), –H160A (A), and –H160D (D) compared with the wild-type channel (H) (n = 3–6). Solid lines represent best fits according to Eq. 2.