Abstract
This paper describes the design, evaluation, and preliminary results of a specialized testing device and surgical protocol to determine translational stiffness of a rabbit knee, replicating the clinical anterior drawer test. Coronal-plane transverse pins are inserted through the rabbit leg, two in the tibia and one in the distal femur, to hold and reproducibly position the leg in the device for tests at multiple time points. A linear stepper motor draws the tibia upward then returns to the home position, and a load cell measures the resisting force; force-displacement knee stiffness is then calculated. Initial evaluation of this testing device determined the effects of preconditioning, intra-operator repeatability, rabbit-to-rabbit variability, knee flexion angle (90° vs. 135°), and anterior cruciate ligament (ACL) sectioning (0%, 25%, 50%, 75%, 100%). Knee stiffness generally decreased as ACL sectioning increased. This testing device and surgical protocol provide an objective and efficient method of determining translational rabbit knee stiffness in vivo, and are being used in an ongoing study to evaluate the effect of knee instability (via partial to complete ACL sectioning) on the development of post-traumatic osteoarthritis.
Keywords: Animal model, anterior cruciate ligament, osteoarthritis, knee laxity, mechanical testing
Introduction
Successful orthopaedic management of articular ligamentous injuries and intra-articular fractures, to prevent or forestall post-traumatic osteoarthritis (PTOA), depends on avoidance of a chronic pathomechanical environment that is deleterious to articular cartilage (Honkonen, 1994; Matta, 1996; Stevens et al., 2001). Chronic pathomechanical residuals of articular injuries include incongruity and instability. It has been proposed that instability leads to abnormal loading on the cartilage, which leads to cartilage degradation, which then leads to PTOA. Clinically, joint instability has consistently been observed to cause PTOA in the hip, knee, and ankle (Delamarter et al., 1990; Lansinger et al., 1986; Letournel, 1980; Matta, 1996; Weber and Simpson, 1985; Yablon, 1989).
Instability has been defined clinically as abnormal motion under normal loading conditions (White et al., 1975). Several animal models have demonstrated that instability leads to joint deterioration. For example, it has been well-established that rabbit knees subjected to complete transection of the anterior cruciate ligament (ACL) will predictably degenerate within several months (Chang et al., 1997; Sah et al., 1997; Yoshioka et al., 1996). However, this instability has not been rigorously quantified. While it has been shown that grossly unstable joints usually develop PTOA, it is plausible that lesser amounts of instability, such as what may occur from a partial ligamentous injury, malalignment, or post-fracture incongruity, may also lead to PTOA. Also, it is possible that below some threshold level of instability there is a reduced chance of onset of PTOA.
To study the pathomechanical relationship between instability and PTOA, it is necessary to characterize the relationship between severity of instability and the speed and severity of the resulting secondary degenerative changes. Clinically, ACL injuries have been particularly closely associated with PTOA (Daniel et al., 1994; Scavenius et al., 1999; Sommerlath et al., 1991). We have developed a rabbit model of gradational knee instability by means of partial to complete sectioning of the ACL. To quantify the resulting increase in joint laxity, we have designed a specialized testing device to measure translational stiffness of the rabbit knee in vivo. The purpose of this study was to validate this device and its corresponding surgical protocol, and to demonstrate its use by measuring stiffness changes resulting from 0%, 25%, 50%, 75%, and 100% ACL sectioning.
Methods
The testing device was designed to measure translational stiffness in New Zealand white rabbit knees, replicating the clinical anterior drawer test. Coronal-plane transverse pins are inserted through the rabbit leg to hold and reproducibly position it in the device for tests at multiple time points. Pin placement and ACL sectioning is performed in a surgical suite using inhalation anesthesia and aseptic techniques, in accordance with our institutional animal care and use committee (IACUC) guidelines. The testing device accommodates rabbit knee flexion angles of 90° and 135°; these angles were chosen because 90° is a typical knee flexion angle used in the clinical anterior drawer test, and 135° is a typical knee flexion angle for sitting rabbits.
Three coronal-plane transverse pins are inserted through the rabbit lower extremity (Figure 1). The initial pin is inserted in the mid-coronal plane of the proximal tibia with freehand technique, placing the pin perpendicular to the long axis of the tibia. The knee is flexed to 90° and an orthogonal drill guide is assembled around the first pin. This drill guide is used to assure that a second pin drilled through the distal tibia, and a third pin drilled through the distal femur, are parallel with the initial proximal tibial pin. The pins are cut to about 10 mm on each side of the limb, and capped with Juergens balls when the pins are not attached to the testing device.
Figure 1.
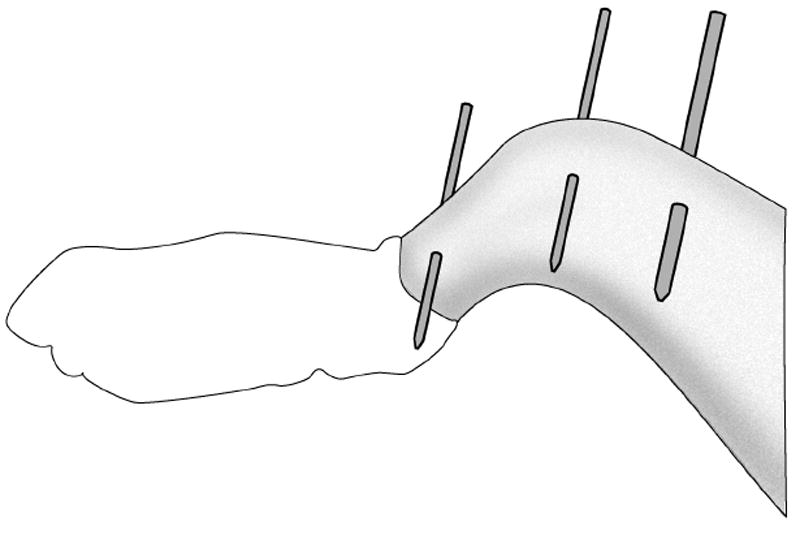
Placement of two tibia pins and one femur pin in rabbit leg. The pins are subsequently cut to about 10mm on each side of the limb. These pins are used to hold and reproducibly position the leg in the knee testing device for tests at multiple time points.
The testing device for measuring rabbit knee joint stiffness (Figure 2) includes a main cradle in which an anesthetized rabbit is placed on its back. The rabbit’s femur is held vertically, and is attached to a stationary femur clamp through the femur pin. The rabbit’s tibia is attached to a moveable tibia clamp through the two tibia pins, with the knee flexion angle set at 90° or 135°. The tibia clamp, and the tibia, are moved anteriorly by a stepper motor, and the resisting force is measured with a load cell. Test control and data acquisition are by a user-written LabView program running on a laptop computer (Figure 3). The testing device draws the tibia upward at 1 mm/sec to a maximum of 3 mm or 75 N (whichever is reached first), then returns to the home position. After the test concludes, force vs. displacement is displayed and saved to a data file.
Figure 2.
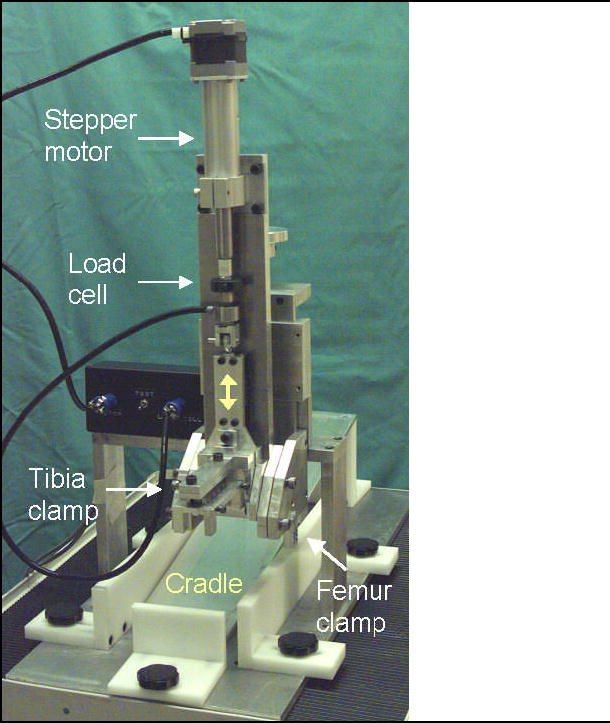
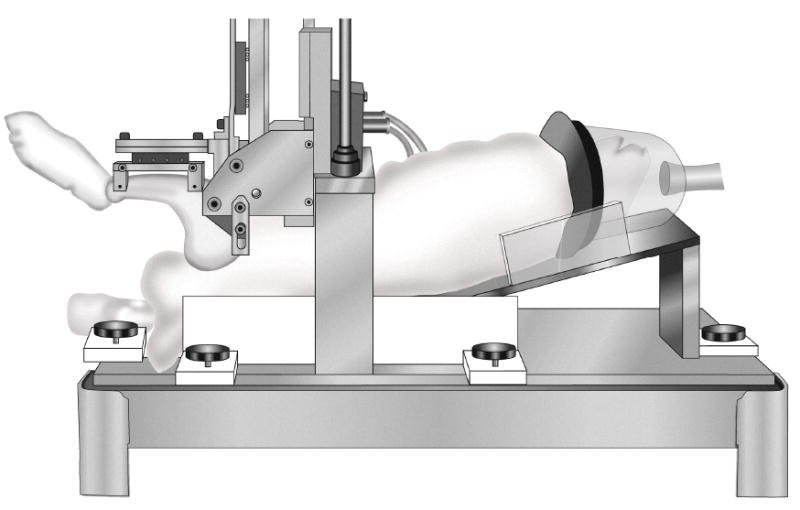
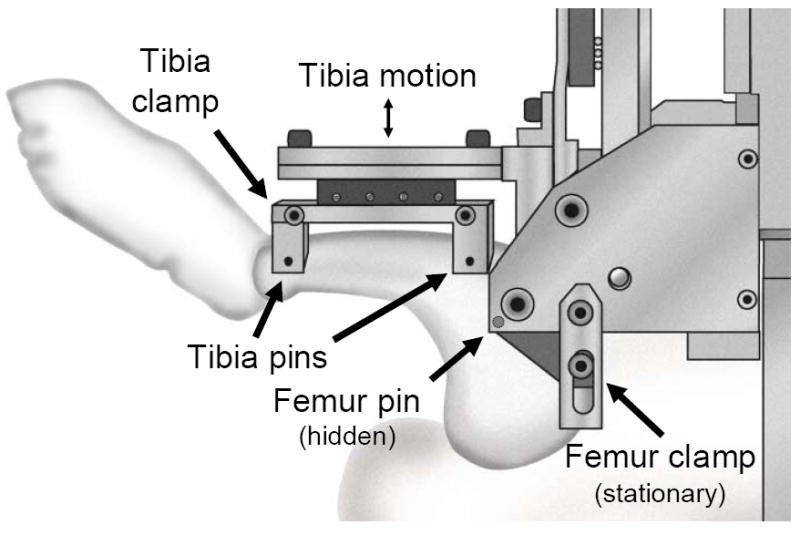
Rabbit knee testing device – a) overview, b) rabbit in place, and c) close-up of rabbit leg in testing device. This testing device measures translational stiffness in rabbit knees, replicating the clinical anterior drawer test. The femur is held vertically, and is attached to a stationary femur clamp through the femur pin. The tibia is attached to a moveable tibia clamp through the two tibia pins, with the knee flexion angle set at 90° (shown) or 135°. The tibia clamp, and the tibia, are moved anteriorly by a stepper motor, then returned to the home position, while force and displacement are recorded.
Figure 3.
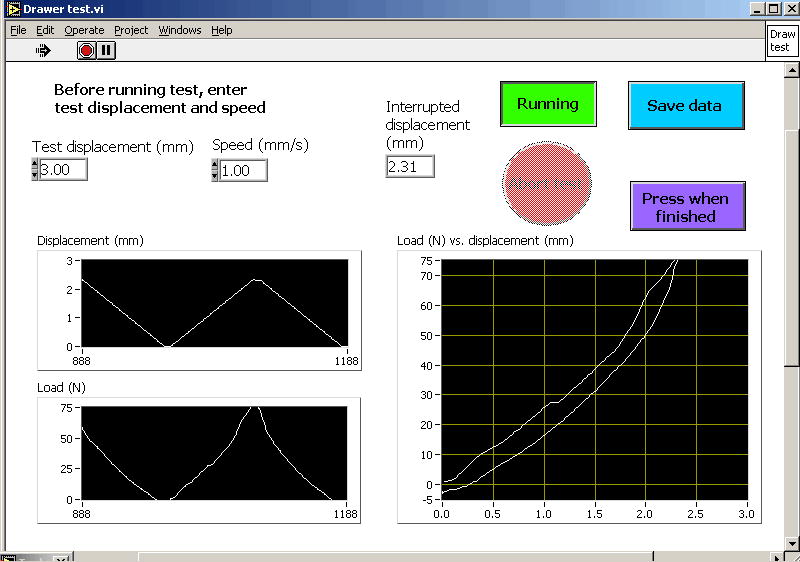
LabView test panel for test control, data display, and data acquisition for the rabbit knee translational drawer test.
Translational stiffness is calculated as the secant slope between 10 N and the maximum force. Postoperative stiffnesses are normalized to the corresponding stiffness for the intact, preoperative condition (with pin placement only) for each rabbit. Data are calculated and analyzed using Excel.
Initial evaluation of this testing device determined the effects of preconditioning, intra-operator re-positioning repeatability, and rabbit-to-rabbit variability, on the knee stiffness of rabbits in the intact, preoperative condition. To evaluate preconditioning, ten consecutive drawer tests were run. To evaluate intra-operator re-positioning repeatability, the operator completely removed and replaced each rabbit in the fixture, for three testing runs.
This testing device is currently being used in an ongoing study to measure the effect of partial to complete ACL sectioning on knee stiffness and subsequent development of osteoarthritis. With the specimen knee hyperflexed, the ACL was isolated by lifting the distal mid-substance anteriorly, and sectioned using a number 11 scalpel blade. The ACL was sectioned by 0% (sham surgery, arthrotomy only), 25%, 50%, 75%, or 100%. The partial sectioning widths (25%, 50%, 75%) were visually estimated by the surgeon, and the sectioning direction was from the medial border of the ACL. Rabbit knee stiffness was measured before and after this surgery. (An additional control group received no knee surgery, just surgical pin placement.) ANOVA and Duncan’s multiple range test were used to determine statistical significance between the ACL sectioning groups (α = 0.05).
Results
The rabbits tolerated the initial surgery and testing well. For the ongoing study, which involves the rabbits surviving to sixteen weeks postoperatively, the animals have tolerated their pins and resumed normal cage activity in the majority of cases. Complications to date (for a current total of 78 rabbits) have been ten tibia fractures (12.8%), three pin-tract infections (3.8%), and one adverse reaction to anesthesia (1.3%).
Four preconditioning cycles were generally sufficient to get repeatable results. Removing and replacing the rabbit in the fixture had little effect on the testing results, with slope variabilities of 4.1% and 0.9% for the 90° and 135° knee flexion angles, respectively. Intact knee stiffness was reasonably consistent between rabbits, with slope variabilities of 24% and 25% for the 90° and 135° knee angles, respectively (n = 76).
The amount of ACL sectioning affected the knee stiffness, with stiffness generally decreasing as the ACL sectioning increased (Figure 4). For the 90° knee angle, the 0% (sham) vs. 25% ACL sectioning results, and the 25% vs. 50% ACL sectioning results, were not significantly different from one another (Figure 4a). For the 135° knee angle, the 0% (sham) ACL sectioning result was not significantly different from the 25% or 50% ACL sectioning results (Figure 4b). Complete ACL sectioning resulted in knee stiffnesses that were an average of 42% and 53% of the corresponding intact specimens, for the 90° and 135° knee angles respectively.
Figure 4.
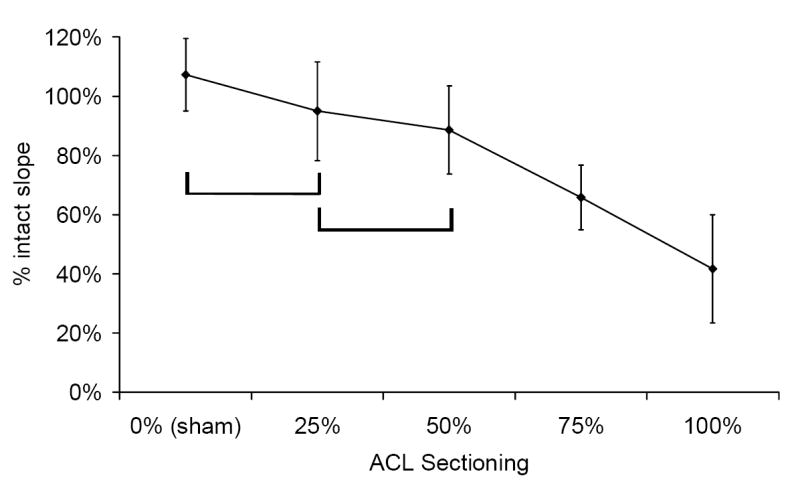
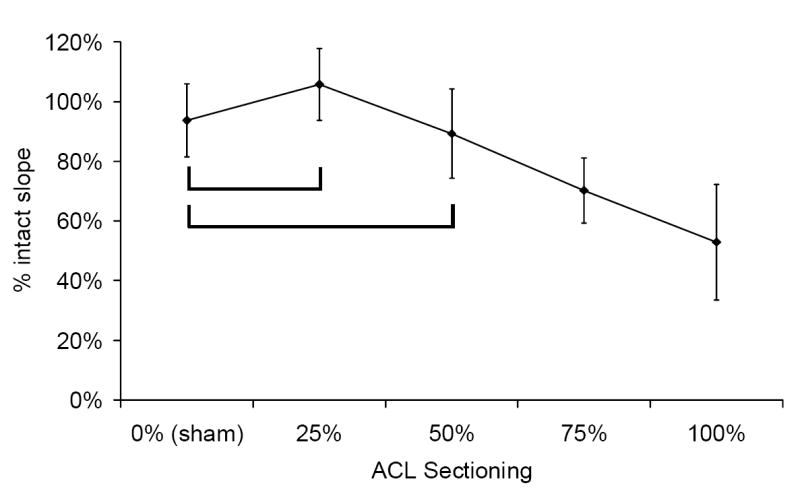
Effect of rabbit knee angle and % ACL sectioning on knee stiffness, for the drawer test at knee angles of a) 90° and b) 135°. Sample size for all sectioning groups was between seven and ten. The brackets indicate groups that were not significantly different from each other (α = 0.05).
Discussion
Articular instability resulting from intra-articular fracture or ligament injury is believed to cause abnormal kinematics, which leads to cartilage degeneration (Harrington, 1979; Lansinger et al., 1986; Matta, 1996; Weber and Simpson, 1985; Yablon and Leach, 1989). To evaluate the pathomechanical link between instability and PTOA, it is necessary to quantify joint stiffness and correlate such a measure with the rate and magnitude of articular degenerative changes.
We have designed and evaluated a testing device and surgical protocol to determine translational stiffness of a rabbit knee, replicating the clinical anterior drawer test. Stiffness results were reproducible, with little intra-observer variability and modest variability between rabbits. Destabilizing the knee by increasing the amount of ACL sectioning decreased the knee stiffness, thus increasing knee laxity. Surgical and postoperative complications have been minimal.
This testing device is currently being used in an ongoing study to assess the relationship between the level of experimentally introduced knee laxity and the subsequent development of osteoarthritis. Different degrees of knee instability are induced by partial to complete ACL sectioning, and the resulting knee stiffness is measured post-surgery and then every four weeks out to sixteen weeks. Changes in knee stiffness are then compared to the presence and severity of osteoarthritic changes that have developed.
In conclusion, this testing device and surgical protocol provide an objective and efficient method of determining translational rabbit knee stiffness in vivo. Such a device will be useful in determining changes in knee stiffness associated with joint laxity, and will facilitate investigations relating instability to PTOA.
Acknowledgments
This study was funded by CDC R49 CCR721745 and by NIH 5 P50 AR048939.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chang D, Iverson E, Schinagl R, Sonoda M, Amiel D, Coutts R, Sah R. Quantitation and localization of cartilage degeneration following the induction of osteoarthritis in the rabbit knee. Osteoarthritis & Cartilage. 1997;5(5):357–72. doi: 10.1016/s1063-4584(97)80039-8. [DOI] [PubMed] [Google Scholar]
- Daniel D, Stone M, Dobson B, Fithian D, Rossman D, Kaufman K. Fate of the ACL-injured patient. A prospective outcome study. American Journal of Sports Medicine. 1994;22(5):632–44. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- Delamarter R, Hohl M, Hopp E., Jr Ligament injuries associated with tibial plateau fractures. Clinical Orthopaedics & Related Research. 1990;250:226–33. [PubMed] [Google Scholar]
- Harrington K. Degenerative arthritis of the ankle secondary to long-standing lateral ligament instability. Journal of Bone & Joint Surgery - American. 1979;61(3):354–61. [PubMed] [Google Scholar]
- Honkonen S. Indications for surgical treatment of tibial condyle fractures. Clinical Orthopaedics & Related Research. 1994;302:199–205. [PubMed] [Google Scholar]
- Lansinger O, Bergman B, Korner L, Andersson G. Tibial condylar fractures. A twenty-year follow-up. Journal of Bone & Joint Surgery - American. 1986;68(1):13–9. [PubMed] [Google Scholar]
- Letournel E. Acetabulum fractures: classification and management. Clinical Orthopaedics & Related Research. 1980;151:81–106. [PubMed] [Google Scholar]
- Matta J. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. Journal of Bone & Joint Surgery - American. 1996;78(11):1632–45. [PubMed] [Google Scholar]
- Sah R, Yang A, Chen A, Hant J, Halili R, Yoshioka M, Amiel D, Coutts R. Physical properties of rabbit articular cartilage after transection of the anterior cruciate ligament. Journal of Orthopaedic Research. 1997;15(2):197–203. doi: 10.1002/jor.1100150207. [DOI] [PubMed] [Google Scholar]
- Scavenius M, Bak K, Hansen S, Norring K, Jensen K, Jorgensen U. Isolated total ruptures of the anterior cruciate ligament – a clinical study with long-term follow-up of 7 years. Scandinavian Journal of Medicine & Science in Sports. 1999;9(2):114–9. doi: 10.1111/j.1600-0838.1999.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Sommerlath K, Lysholm J, Gillquist J. The long-term course after treatment of acute anterior cruciate ligament ruptures. A 9 to 16 year followup. American Journal of Sports Medicine. 1991;19(2):156–62. doi: 10.1177/036354659101900211. [DOI] [PubMed] [Google Scholar]
- Stevens D, Beharry R, McKee M, Waddell J, Schemitsch E. The long-term functional outcome of operatively treated tibial plateau fractures. Journal of Orthopaedic Trauma. 2001;15(5):312–20. doi: 10.1097/00005131-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Weber B, Simpson L. Corrective lengthening osteotomy of the fibula. Clinical Orthopaedics & Related Research. 1985;199:61–7. [PubMed] [Google Scholar]
- White A, 3rd, Johnson R, Panjabi M, Southwick W. Biomechanical analysis of clinical stability in the cervical spine. Clinical Orthopaedics & Related Research. 1975;109:85–96. doi: 10.1097/00003086-197506000-00011. [DOI] [PubMed] [Google Scholar]
- Yablon I, Leach R. Reconstruction of malunited fractures of the lateral malleolus. Journal of Bone & Joint Surgery - American. 1989;71(4):521–7. [PubMed] [Google Scholar]
- Yoshioka M, Coutts R, Amiel D, Hacker S. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis & Cartilage. 1996;4(2):87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]


