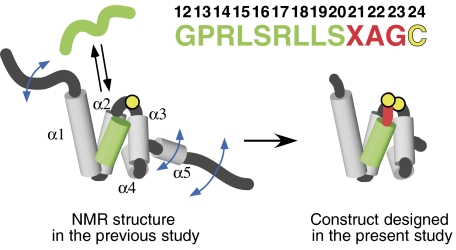
Figure 1.
Design of the Tom20–presequence complex. The solution structure of the cytosolic domain of Tom20, previously determined by NMR (Abe et al, 2000), consists of five α-helices, α1–α5 (gray), with flexible N- and C-terminal segments. The presequence associates with and dissociates from Tom20 rapidly in solution. The presequence from aldehyde dehydrogenase (ALDH) is shown in green. The presequence peptide has no tertiary structure in the free state, but assumes an α-helical conformation in the bound state. Note that the numbering starts at 12, since this sequence corresponds to the C-terminal half of the ALDH presequence (Farres et al, 1988). A cysteine residue (yellow) was attached to the C terminus after the three-residue linker (red). The intermolecular disulfide bond was formed with the single cysteine residue of Tom20 (Cys100, yellow circle), and the mobile α5 and two flexible termini were removed, to prepare the Tom20–presequence complexes for crystallization and the NMR relaxation study.