Abstract
Although following another person's gaze is essential in fluent social interactions, the reflexive nature of this gaze-cuing effect means that gaze can be used to deceive. In a gaze-cuing procedure, participants were presented with several faces that looked to the left or right. Some faces always looked to the target (predictive-valid), some never looked to the target (predictive-invalid), and others looked toward and away from the target in equal proportions (nonpredictive). The standard gaze-cuing effects appeared to be unaffected by these contingencies. Nevertheless, participants tended to choose the predictive-valid faces as appearing more trustworthy than the predictive-invalid faces. This effect was negatively related to scores on a scale assessing autistic-like traits. Further, we present tentative evidence that the “deceptive” faces were encoded more strongly in memory than the “cooperative” faces. These data demonstrate the important interactions among attention, gaze perception, facial identity recognition, and personality judgments.
When people observe someone looking in a particular direction, their attention is shifted to the same location in space (Driver et al., 1999; Friesen & Kingstone, 1998; Hietanen, 1999; Langton & Bruce, 1999). This shift of attention results in reaction time (RT) advantages for targets appearing at that location, compared with targets at other locations. In development, this joint attention is pivotal for language and theory-of-mind development (Charman, 2003; Moore & Dunham, 1995). Because reading the mind from the eyes is so important to social interactions (Baron-Cohen, 1995, 2000), gaze behavior may play a key role in personality evaluation and person perception (Hood, Macrae, Cole-Davies, & Dias, 2003; Kleinke, 1986; Macrae, Hood, Milne, Rowe, & Mason, 2002; Mason, Hood, & Macrae, 2004; Mason, Tatkow, & Macrae, 2005).
When encountering another individual, one forms a stronger memory trace of that person if his or her gaze is directed toward oneself than if it is directed elsewhere (e.g., Mason et al., 2004). Further, people find individuals who make direct eye contact more trustworthy and more attractive than individuals who do not make eye contact (e.g., Mason et al., 2005). However, it should be noted that prolonged direct gaze can be seen as threatening (Argyle & Cook, 1976) and increases arousal (Nichols & Champness, 1971). Interestingly, even though gaze direction and face identity appear to be encoded in different neural structures (superior temporal sulcus and fusiform gyrus, respectively; e.g., Hoffman & Haxby, 2000), fusiform face-identification processing can be modulated by gaze direction, with activation increasing when gaze is directed toward the viewer (e.g., Pelphrey, Singerman, Allison, & McCarthy, 2003).
These effects show that direct gaze influences person perception. However, in addition to indicating an individual's intentions regarding interactions with other members of a social group, gaze behavior is an excellent cue regarding the object a person is currently interested in. An animal that becomes aware of the presence of an interesting object—for example, a food source, a predator, or the dominant member of the group—will look toward that object. The knowledge that other group members' orienting is driven to important objects enables animals with gaze-following abilities to use gaze cues to become aware of events and objects that they otherwise would not notice.
Even in humans, shifts in joint attention that are evoked by gaze cues seem impervious to the nature of the face producing the gaze shift. This makes it difficult to conclude that these shifts have any impact on, or are influenced by, person perception processes. Whether a cartoon face (e.g., Friesen & Kingstone, 1998) or a single photograph of a face produces the cue seems to make little difference (e.g., Driver et al., 1999). Furthermore, Frischen and Tipper (2004) showed that gaze cuing is not modulated by whether the same face is presented for hundreds of trials or a different face appears on every trial, suggesting that novelty of face identity has no influence on joint attention. Even more striking, the emotional expression, such as fear or anger, of the face producing the gaze shift has no effect on the magnitude of cuing (Hietanen & Leppanen, 2003), except in participants with high state and trait anxiety (Mathews, Fox, Yiend, & Calder, 2003).
Even though the properties of a face appear to have no influence on the rapid and automatic shifts of attention evoked by gaze shifts, it does not follow that the opposite relation cannot be found. That is, a shift in joint attention triggered by gaze may influence the perception of the individual producing the gaze shift. Outside the laboratory, gaze direction is usually a highly accurate indicator of the location of interesting objects; even young children understand this. For example, Friere, Eskritt, and Lee (2004) have shown that from the age of 4 years, children use gaze direction to locate objects despite conflicting verbal information (e.g., an adult looks to a hidden object but states, “I don't know where it is”). However, during competition between individuals, gaze shifts can be used to deceive (see Emery, 2000, for review). Consider, for example, a basketball player's feint; the player gazes to the left, but then makes a quick pass to the right. To use such tactics, the player must know that automatic encoding of gaze direction will lead opponents not only to follow the gaze cue, but also to predict a leftward action. Detecting such deceivers is important, and indeed, previous studies have shown that the faces of deceivers are encoded more strongly into memory than those of cooperators (Yamagishi, Tanida, Mashima, Shimoma, & Kanazawa, 2003). Further, trustworthiness judgments of faces are affected in simple computerized games in which faces can be associated with either cooperative or deceptive behavior, such that cooperative faces are judged to be more trustworthy than deceptive ones (Singer, Kiebel, Winston, Dolan, & Frith, 2004).
In the current study, we manipulated the predictability of gaze cuing. Some faces were completely unpredictive, in that half the time they looked toward the subsequent location of a target (valid trials) and half the time they looked to the opposite side of space (invalid trials). This is the usual gaze-cuing procedure. Other faces always gazed toward the subsequent location of the target, and so they were “cooperative.” A final group of faces always looked away from the subsequent location of the target, and so they were “deceivers.”
Given previous research, we predicted that the nature of the individuals making the gaze shift would not clearly influence participants' rapid and automatic shifts of attention. That is, we expected that the gaze-cuing effects would not differ consistently across the different types of stimulus faces. However, the relation between gaze-cuing contingency and face identity might nevertheless be encoded and influence person perception. Thus, if people link helpful and unhelpful gaze-evoked attention shifts to the identity of the persons who produce these gaze cues, then the faces that always cooperated would seem more trustworthy than the faces that never looked toward the correct target location. If, however, such gaze-cuing episodes occur completely separately from person-perception processes, then personality judgments would be unaffected by whether the faces were cooperators or deceivers. For half our subjects, we also investigated whether the deceptive faces made more of an impact on memory than the cooperative faces (Yamagishi et al., 2003). If our predictions were borne out by the data, this study would provide the first evidence that the attention shifts induced by observing another person's averted gaze have consequences regarding the evaluation of that person's personality.
METHOD
Participants
Forty adults (8 males) were recruited from the undergraduate participant panel at the School of Psychology, University of Wales, Bangor, United Kingdom. Their mean age was 20.3 years (SD = 2.1 years), and their mean Autism-Spectrum Quotient (AQ; Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001) score was 15.3 out of 50 (SD = 6.4), well within the normal range. All were naive as to the purpose of the experiment in general, and therefore naive to the predictive nature of some of the faces' gaze cues. Informed consent was obtained, and participants received course credit for their participation.
Stimuli
Forty unfamiliar faces were used as cue stimuli. Three versions of each face were produced, one with gaze straight ahead (i.e., so it would look directly at the participant when presented at the middle of the screen), one with the pupils averted leftward, and another with the pupils averted rightward. The 40 faces were split into 10 groups of 4 faces that, within each group, were matched for gender, ethnicity, and approximate age. The target stimuli consisted of 40 household items, 20 that could generally be found in the garage (household tools) and 20 that were kitchen items. These stimuli could randomly appear in their original orientation (the way they appeared when collected from the Internet) or subjected to a left-right mirror-image reversal and in one of four colors: red, blue, green, or yellow. We included this number of target stimuli to introduce a degree of variety into the task.
Design
There were two within-subjects factors. The first was validity: Valid trials were those on which the eyes looked toward the same side of the screen as the target location, and invalid trials were those on which the eyes looked to the side opposite to the subsequent target. The second within-subjects factor was face type: Each of the 10 face groups contained four faces, two that were nonpredictive of target location and two that were predictive. Nonpredictive faces looked toward the target location as many times as they looked away from the target location. Hence, across the 12 times that such a face appeared, it looked 6 times toward the target and 6 times away from the target. Of the two predictive faces in each group, one was predictive-valid, looking toward the target location on each of the 12 trials in which it appeared, and the other was predictive-invalid, looking 12 times to the side opposite the target location. For each participant, assignment of the four faces in each group to the nonpredictive, predictive-valid, and predictive-invalid conditions was randomized. The order of face presentation was randomized within sets of 40 trials. Task was varied as a between-subjects factor, with 20 participants responding to the category of the target object (kitchen or garage), and 20 responding simply to the object's location (left or right).
Procedure
Gaze-Cuing Procedure
Participants fixated the center of the screen and were required to respond quickly and accurately to the target. In the categorization group, the “h” and space-bar keys were used as response keys. Because the “h” key is directly above the space bar, this up/down response was orthogonal to the left/right location of the target. Which key corresponded to which category was counterbalanced across participants. In the localization group, the “z” and “/” keys, on the left and right sides of the keyboard, were used as response keys. On each trial, a fixation cross was presented for 600 ms, and then a face with direct gaze appeared in the center of the screen for 1,500 ms. Next, the eyes moved to the left or right, and 500 ms later the target appeared. This display containing the face and target remained on the screen until the participant responded or 2,500 ms had elapsed and was then replaced by a blank screen that remained for 1,500 ms before the next trial (see Fig. 1). Participants completed 480 trials, over six blocks. In each block, the 40 faces were presented twice (see the previous paragraph). Altogether, there were 120 predictive-valid, 120 predictive-invalid, 120 nonpredictive-valid, and 120 nonpredictive-invalid trials. Prior to the experiment, participants also took part in 30 practice trials that had the same targets, but different faces.
Fig. 1.
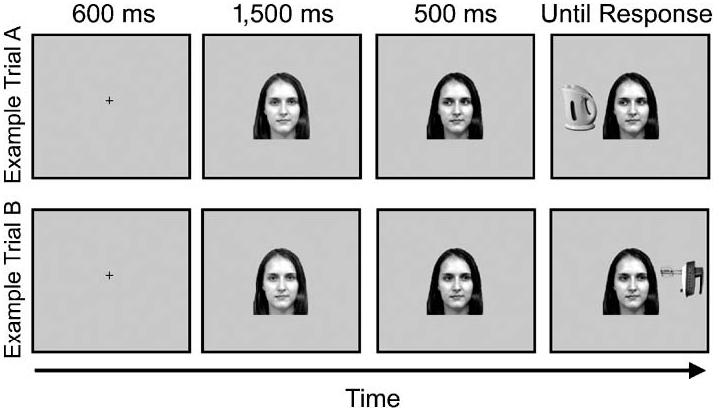
Examples of the time course of two trials containing a predictive-valid face. Throughout an experimental session, this person always looked to the subsequent target location. For other participants, this person always looked away from the target or was nonpredictive of target location.
Face-Choice Procedure
At the end of the experiment, the predictive-valid and predictive-invalid faces from each of the 10 groups of faces were presented as a pair, and participants had to choose the one they felt was more trustworthy. One face appeared to the left of the center of the screen, and the other to the right of the center. After 3,000 ms, the number prompts “1” and “2” appeared above the left and right faces, respectively. At this point, the participants were free to make their choice by hitting the “1” or “2” key on the keyboard. A 2,000-ms blank screen followed response. The order in which the pairs were presented and the side each face type appeared on were randomized. The nonpredictive faces were not presented during this phase of the experiment.
The localization group made two additional judgments after the trustworthiness judgment. Participants saw the same pairs of faces again (the order of the pairs and the positions of the faces were again randomized) and were asked which face in each pair they “preferred.” Then, the participants were shown the faces again and asked to choose the member of each pair that they thought had been “presented most often during the experiment,” even though in reality all the faces had been presented equally often.1
AQ
Finally, participants completed the AQ, devised by Baron-Cohen et al. (2001) as a measure of autistic traits in the normal population. We have previously found that AQ score can be related to gaze-cuing effects (Bayliss, di Pellegrino, & Tipper, 2005; Bayliss & Tipper, 2005). We predicted that the personality judgments in this experiment would relate to AQ scores, in that lower scores (i.e., fewer autistic-like traits) would be associated with greater sensitivity to the social contingencies in the gaze cues.
RESULTS
Gaze Cuing
Table 1 presents the mean RTs and error rates in the gaze-cuing phase of the experiment. We report statistical analyses using prep (probability of replication) in place of p values (see Killeen, 2005). High prep values correspond to low p values (e.g., p = .05 corresponds to prep ≈ .917).
TABLE 1.
Mean Reaction Times and Error Rates
| Reaction time (ms) |
Error rate |
|||
|---|---|---|---|---|
| Task and condition | Mean | SD | Mean | SD |
| Localization | ||||
| Predictive-valid | 328 | 62.0 | 0.38 | 0.50 |
| Predictive-invalid | 337 | 66.3 | 1.00 | 0.80 |
| Nonpredictive-valid | 327 | 60.0 | 0.71 | 1.00 |
| Nonpredictive-invalid | 338 | 69.2 | 0.83 | 0.60 |
| Categorization | ||||
| Predictive-valid | 702 | 95.2 | 7.90 | 7.20 |
| Predictive-invalid | 715 | 95.9 | 7.00 | 6.90 |
| Nonpredictive-valid | 701 | 91.4 | 5.80 | 5.80 |
| Nonpredictive-invalid | 712 | 95.0 | 8.20 | 6.90 |
Errors
A repeated measures analysis of variance (ANOVA) was performed on the mean error percentages. Within-subjects factors were face type (predictive or nonpredictive face) and validity (valid or invalid trial); task (categorization or localization) was a between-subjects factor. The main effect of task was significant, F(1, 38) = 20.3, prep = .998, ηp2 = .348, with more errors in the categorization group (7.2%) than in the localization group (0.74%). There was also a significant cuing effect, with fewer errors in valid trials (3.7%) compared with invalid trials (4.2%), F(1, 38) = 11.1, prep = .986, ηp2 = .226. The main effect of face type was nonsignificant, F(1, 19) < 1. However, the Face Type × Validity interaction was also significant, F(1, 38) = 4.12, prep = .918, ηp2 = .098, as was the three-way Task × Face Type × Validity interaction, F(1, 38) = 8.05, prep = .972, ηp2 = .175. That is, in the categorization task, there tended to be more cuing for nonpredictive than predictive faces, whereas the opposite trend was observed for target localization. However, the error rate was generally low, so the importance of these effects may be limited.
RT
Trials with RTs more than 2 standard deviations above or below a participant's mean RT were removed from analysis (4.2% of trials in the localization task and 4.7% in the categorization task). The remaining correct RTs contributed to cell means and were submitted to a mixed-factors ANOVA. Task was a between-subjects factor, and face type and validity were within-subjects factors. There were significant effects of both task, F(1, 38) = 220, prep > .999, ηp2 = .852, and validity, F(1, 38) = 24.8, prep = .999, ηp2 = .395; participants were faster in the localization task (332 ms) than in the categorization task (708 ms) and were also quicker to respond on valid (515 ms) than on invalid (526 ms) trials (see Table 1). No other effects approached significance (Fs < 1). Cuing effects were found in both face-type conditions for both groups (ts > 2.42, preps > .917).
In general, gaze-cuing effects were approximately uniform across conditions and tasks. That is, gaze contingency (predictive vs. nonpredictive) had little impact on gaze-evoked shifts of attention: The rapid and automatic gaze shifts that speed up processing of targets were immune to the properties of person identity. However, it is possible that the effects of predictive gaze contingencies emerged as the experiment progressed. Therefore, we isolated the final third of the experiment for further analysis. No effect of face type was detected in either error rates or RTs for either task. Further, the unexpected and difficult-to-account-for interaction of task, face type, and validity observed in error rates overall was no longer present after participants were extensively exposed to the face cuing contingencies.
Face Choices
Participants chose predictive-valid faces as more trustworthy than their age-, gender-, and ethnicity-matched predictive-invalid counterparts an average of 57% of the time. A one-sample t test demonstrated that this percentage was significantly different from the chance level of 50%, t(39) = 2.23, prep = .936, d = 0.35, two-tailed. Also, participants in the localization group tended to choose the predictive-valid faces as preferable to the predictive-invalid faces (57% of trials). This bias approached significance, t(19) = 2.06, prep = .914, d = 0.46, two-tailed. However, these participants tended to feel that the predictive-invalid faces were the ones that had been presented more often during the gaze-cuing phase (the predictive-valid faces were chosen on 44% of trials). Although this bias was weaker than the bias for preference and trustworthiness judgments, t(19) = −1.85, prep = .891, d = 0.41, two-tailed, the important point is that the trend is in the direction opposite to the trend for the other two measures (see Fig. 2).
Fig. 2.
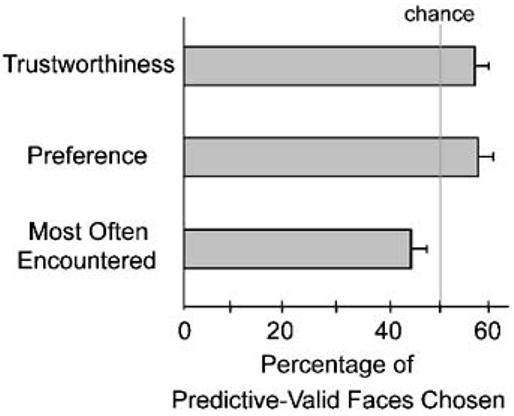
Percentage of predictive-valid (i.e., cooperative) faces chosen over predictive-invalid (i.e., deceptive) faces, for each of the following three questions: “Which face do you find more trustworthy?” (n = 40), “Which face do you prefer?” and “Which face do you think was presented most often during the experiment?” (both ns = 20). Chance performance was 50%.
We also calculated two-tailed Pearson's correlations using these data. The percentage of predictive-valid faces chosen on each of the three measures was correlated with both AQ scores and cuing magnitudes (i.e., invalid trials minus valid trials) for predictive and nonpredictive faces. Although the scores on the choice measures did not correlate significantly with cuing effects on errors ( preps < .852), there were several interesting correlations involving RT cuing effects.
First, the percentage of times a participant chose the predictive-valid face as more trustworthy than the predictive-invalid face was significantly positively related to magnitude of the cuing effect from the predictive faces, r = .418, n = 40, prep = .972. Hence, the greater the cuing elicited by the predictive faces (i.e., the greater impact these cues had on the attention system), the stronger the effect on perceived trustworthiness was. However, as expected, cuing from nonpredictive faces (i.e., those that were not evaluated for trustworthiness after the cuing phase) was not significantly related to how many predictive-valid faces were chosen as trustworthy, r = .060, n = 40, prep = .603. The preference measure was not significantly correlated with cuing for either type of face (preps < .668). Finally, in contrast with trustworthiness, how often a participant chose predictive-valid faces as being presented more often than predictive-invalid faces was negatively correlated with overall cuing magnitude, r = −.467, n = 20, prep = .929. That is, the larger the magnitude of a participant's overall cuing effects, the less likely he or she was to choose predictive-valid faces as having been presented more often than predictive-invalid faces. Hence, the stronger the cuing effect, the stronger the memory trace produced by the deceptive faces.
The only other correlation to reach significance was the negative correlation between score on the AQ and score on the trustworthiness measure, r = −.372, n = 40, prep = .953. Thus, higher scores on the AQ (i.e., more autistic-like traits) were associated with a reduced tendency to choose the predictive-valid faces as more trustworthy than the predictive-invalid faces. Although AQ score was not significantly related to overall cuing effects or to the other measures ( preps < .828), this is certainly an intriguing result, suggesting that differences in sensitivity to social cues in participants in the normal population are a function of autistic-like traits (see Bayliss et al., 2005; Bayliss & Tipper, 2005).
DISCUSSION
This study investigated whether multiple exposures to faces that consistently looked toward the target location (predictive-valid, or “cooperative,” faces) or consistently looked away from the target location (predictive-invalid, or “deceptive,” faces) would result in participants choosing the former as more trustworthy than the latter. This effect was demonstrated, and there was evidence for a general preference for the cooperative over the deceptive faces. The idea that deceptive faces are encoded into memory more deeply than cooperative faces (e.g., Yamagishi et al., 2003) was tentatively supported by a trend toward the deceptive faces being chosen more often than the cooperative faces as having been presented more often during the experiment, even though the two kinds of faces had been presented an equal number of times. This result demonstrates that the trustworthiness and preference effects were not solely due to a response bias favoring predictive-valid faces. Further, during casual debriefing, very few participants mentioned the face-cue contingencies, which suggests that for the majority of participants, the personality judgments were implicitly affected by the gaze cuing.
In general, the strength of the gaze-evoked attention shift (cuing effect) was not affected by the contingencies associated with the different faces. Certainly, although one would expect effects of contingencies to have emerged in the last third of the experiment, they did not. Thus, the attention system seems to act on gaze cues as if blind to the identity of the face. This finding supports evidence suggesting that the networks responsible for gaze processing and face-identity processing are independent (Hoffman & Haxby, 2000). That is, the rapidly evoked shifts of attention mediated by superior temporal sulcus and parietal networks are not affected by the acquired personality attributes of different faces.
However, our data support the opposite relationship. That is, direction of gaze can influence person perception (e.g., Mason et al., 2005). Thus, the basic finding that the cooperative (predictive-valid) faces were perceived as more trustworthy than the deceptive (predictive-invalid) faces demonstrates that gaze-evoked attention shifts can influence person perception, even in a fairly simple cuing procedure. Further, the results suggest that the history of another person's (fairly innocuous) behavior can influence an observer's evaluation of that person's character. The cuing effects observed here were not large (e.g., mean cuing effect = 11 ms). Nevertheless, over 12 exposures of a face, these small differences seem to have been enough to influence person perception. This demonstrates how important gaze behavior and gaze monitoring are in social groups, and in the interactions between individuals.
That gaze-evoked shifts of attention were related to subsequent person assessments was further revealed by the significant positive correlation between the magnitude of the cuing from predictive faces and the percentage of cooperative faces chosen as more trustworthy than deceptive faces. Thus, on average, if a participant's attention was not cued very strongly by the predictive faces, then that person did not show a bias in his or her trustworthiness judgments. Such individual differences in sensitivity to social cues were further revealed in the assessment of autistic traits (see also Bayliss et al., 2005; Bayliss & Tipper, 2005). AQ score correlated negatively with the trustworthiness effect, revealing that the fewer autistic-like traits participants reported, the more likely they were to detect the gaze contingencies and hence to choose cooperative faces as trustworthy.
In conclusion, although previous studies have shown that gaze can influence person perception (Mason et al., 2005), it was unclear what role attention shifts in averted-gaze stimuli might have in personality evaluation, considering the hypothesized independence of gaze and identity perception (Hoffman & Haxby, 2000). Our results indicate that although gazed-evoked cuing effects seem to be independent of the identity of the face producing the cue (cf. Frischen & Tipper, 2004), a neural system sensitive to reward contingencies, operating in parallel, attaches positive and negative affective tags to faces that cooperate or deceive, respectively. This results in more positive appraisal of cooperative faces, even though deceptive faces appear to be more strongly encoded in memory. In humans' complex, competitive social environment, such a system is vital, to facilitate the identification of cheaters and cooperators from subtle behavioral cues.
Acknowledgments
We wish to thank Satoshi Endo, Victoria Greaves, Julia Gomez, and Keri Winter for assistance with stimulus production and pilot testing. This work was supported by a Wellcome Programme grant awarded to Steven P. Tipper, and by an Economic and Social Research Council (UK) Postdoctoral Fellowship grant to Andrew Bayliss.
Footnotes
Initially, we were unaware of the article by Yamagishi et al. (2003), which reported better implicit memory for faces of deceptive individuals than of cooperative individuals. Only during testing did we realize the potential importance of introducing a measure of the impact of deceptive behavior on memory for faces. We felt that asking a question that did not reveal the critical manipulation in this study to these participants would enable us to investigate implicit memory for the faces in the experiment.
REFERENCES
- Argyle M, Cook M. Gaze and mutual gaze. Cambridge, England: Cambridge University Press; 1976. [Google Scholar]
- Baron-Cohen S. The eye direction detector (EDD) and the shared attention mechanism (SAM): Two cases for evolutionary psychology. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 41–59. [Google Scholar]
- Baron-Cohen S. The cognitive neuroscience of autism: Evolutionary approaches. In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; 2000. pp. 1249–1257. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high functioning autism, males and females, scientists and mathematicians. Journal of Autism & Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, di Pellegrino G, Tipper SP. Sex differences in eye gaze and symbolic cueing of attention. Quarterly Journal of Experimental Psychology. 2005;58A:631–650. doi: 10.1080/02724980443000124. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, Tipper SP. Gaze and arrow cueing of attention reveals individual differences along the autism-spectrum as a function of target context. British Journal of Psychology. 2005;96:95–114. doi: 10.1348/000712604X15626. [DOI] [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society of London B. 2003;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6:509–540. [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience & Biobehavioral Reviews. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Friere A, Eskritt M, Lee K. Are eyes windows to a deceiver's soul? Children's use of another's eye gaze cues in a deceptive situation. Developmental Psychology. 2004;40:1093–1104. doi: 10.1037/0012-1649.40.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5:490–495. [Google Scholar]
- Frischen A, Tipper SP. Orienting attention via observed gaze shift evokes longer-term inhibitory effects: Implications for social interactions, attention and memory. Journal of Experimental Psychology: General. 2004;133:516–533. doi: 10.1037/0096-3445.133.4.516. [DOI] [PubMed] [Google Scholar]
- Hietanen JK. Does your gaze direction and head orientation shift my visual attention? NeuroReport. 1999;10:3443–3447. doi: 10.1097/00001756-199911080-00033. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Leppanen JM. Does facial expression affect attention orienting by gaze direction cues? Journal of Experimental Psychology: Human Perception and Performance. 2003;29:1228–1243. doi: 10.1037/0096-1523.29.6.1228. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hood BM, Macrae CN, Cole-Davies V, Dias M. Eye remember you: The effects of gaze direction on face recognition in children and adults. Developmental Science. 2003;6:67–71. [Google Scholar]
- Killeen PS. An alternative to null-hypothesis significance tests. Psychological Science. 2005;5:345–353. doi: 10.1111/j.0956-7976.2005.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinke CL. Gaze and eye contact: A research review. Psychological Bulletin. 1986;100:78–100. [PubMed] [Google Scholar]
- Langton SRH, Bruce V. Reflexive visual orienting in response to the social attention of others. Visual Cognition. 1999;6:541–567. [Google Scholar]
- Macrae CN, Hood BM, Milne AB, Rowe AC, Mason MF. Are you looking at me? Eye gaze and person perception. Psychological Science. 2002;13:460–464. doi: 10.1111/1467-9280.00481. [DOI] [PubMed] [Google Scholar]
- Mason MF, Hood BM, Macrae CN. Look into my eyes: Gaze direction and person memory. Memory. 2004;12:637–643. doi: 10.1080/09658210344000152. [DOI] [PubMed] [Google Scholar]
- Mason MF, Tatkow EP, Macrae CN. The look of love: Gaze shifts and person perception. Psychological Science. 2005;16:236–239. doi: 10.1111/j.0956-7976.2005.00809.x. [DOI] [PubMed] [Google Scholar]
- Mathews A, Fox E, Yiend J, Calder A. The face of fear: Effects of eye gaze and emotion on visual attention. Visual Cognition. 2003;10:823–835. doi: 10.1080/13506280344000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hove, England: Erlbaum; 1995. [Google Scholar]
- Nichols KA, Champness BG. Eye gaze and the GSR. Journal of Experimental Social Psychology. 1971;7:623–626. [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: The influence of context. Neuropsychologia. 2003;41:156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41:653–662. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Tanida S, Mashima R, Shimoma E, Kanazawa S. You can judge a book by its cover: Evidence that cheaters may look different from cooperators. Evolution and Human Behavior. 2003;24:290–301. [Google Scholar]


