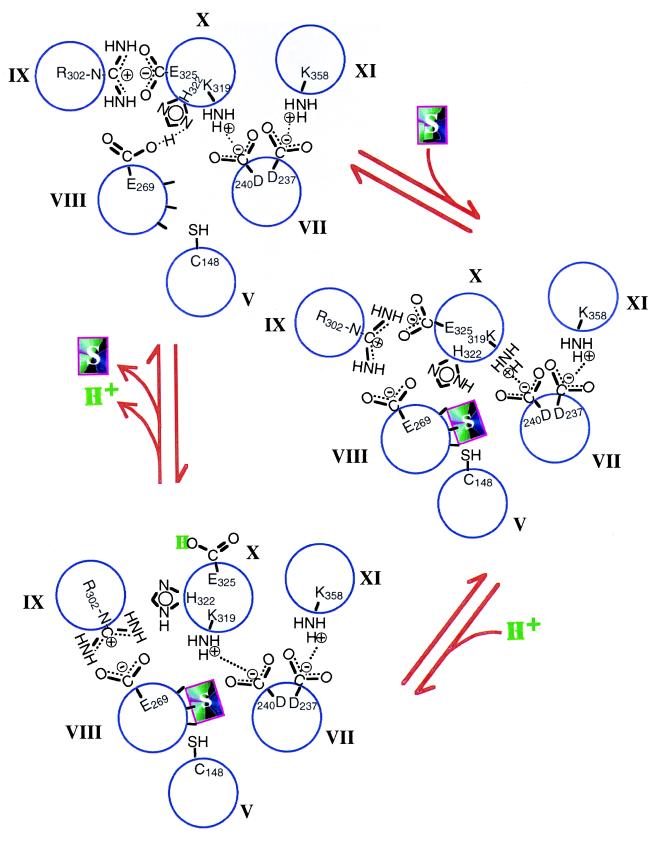
Figure 2.
Proposed mechanism for energy coupling in lac permease. Packing of helices V and VII–XI and important side-chain interactions in the absence of substrate (Upper Left) are based on site-directed excimer fluorescence, which demonstrates that helix VIII (Glu-269) is close to helix X (His-322), helix IX (Arg-302) is close to helix X (Glu-325), and that helix X is in α-helical conformation. The presence of two pairs of charged residues that interact functionally—Asp-237 (helix VII) with Lys-358 (helix XI) and Asp-240 (helix VII) with Lys-319 (helix X)—demonstrates that helix VII is close to helices X and XI. The spatial relationships have been confirmed by engineering divalent metal-binding sites (bis-His residues) within the permease. In addition, mAb 4B11 has been shown to bind to the last two cytoplasmic loops. Site-directed chemical cleavage studies support the positioning of helix X next to helices VII and XI and indicate further that helix V is in close proximity to helices VII and VIII. The relationship between helices V, VII, and VIII has been confirmed by site-directed spin labeling and thiol crosslinking experiments. Upon substrate binding (Lower Right), the interaction between His-322 and Glu-269 is disrupted, leading to the changes in side-chain interactions described in the text. Although not shown, site-directed thiol crosslinking studies demonstrate that helix I is close to helices V and VI, helix II is close to helices VII and XI, and helix VI is close to helices V and VIII.