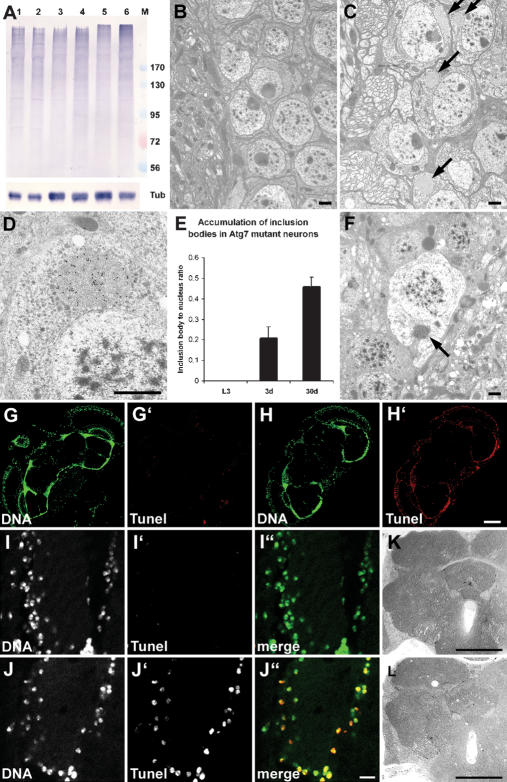
Figure 4.
Ubiquitinated proteins accumulate in Atg7 mutant brains, associated with progressive neurodegeneration. (A) A Western blot shows accumulation of ubiquitinated high-molecular-weight proteins in aging Atg7d77 fly heads. No difference is seen between control (lane 1) and mutant (lane 2) late L3 larval samples. Accumulation of ubiquitinated proteins is already visible in 3-d-old Atg7d77 adult head extracts (lane 4, cf. control in lane 3), and becomes very obvious by day 30 (lane 6, cf. control in lane 5). Numbers next to the marker (M) refer to molecular weights in kilodaltons. β-Tubulin is shown as a loading control. (B–F) Electron micrographs reveal the presence of inclusion bodies in 30-d-old Atg7d77 (arrows in C), but not in control neurons (shown in B). (D) Immunoelectron microscopy confirms that these inclusion bodies contain ubiquitinated proteins. E shows progressive accumulation of aberrant protein aggregates in Atg7d77 mutants: No inclusion bodies are seen in late third instar larval brains, while they appear in neurons of 3-d-old males, and further accumulate by age 30 d. (F) Dead neurons are readily identified in 30-d-old mutant brains, many still containing ubiquitin-positive inclusion bodies in the degenerating cytoplasm (arrow). (G–J) TUNEL staining of horizontal brain sections shows widespread DNA fragmentation in Atg7d77 brains. (H,J) Most cells show DNA fragmentation in 30-d-old Atg7d77 brains, (G,I) while no staining is seen in controls. Moderate vacuolization is seen in 30-d-old Atg7d77 mutant brains (L), while no sign of degeneration is observed in controls (K). Bars: B–F, 1 μm; I,J, 10 μm; G,H,K,L, 150 μm. (B,G,I,K) Control: CG5335d30/Atg7d14. (C,D,F,H,J,L) Atg7d77: Atg7d77/Atg7d14.