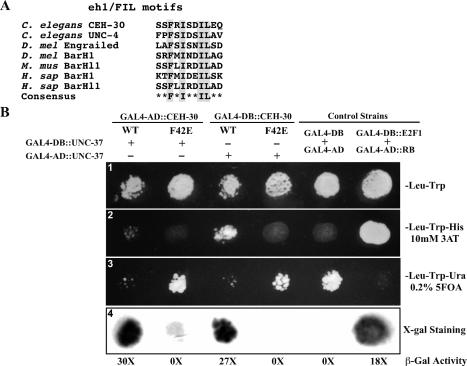
Figure 5.
CEH-30 and UNC-37 interact through the eh1/FIL motif. (A) Alignment of the eh1/FIL motifs in various proteins from C. elegans, Drosophila (D. Mel), mouse (M. Mus), and human (H. sap). Highly conserved amino acid residues are highlighted. (B) Yeast two-hybrid assays. (Panel 1) Yeast transformants expressing CEH-30 and UNC-37 GAL4 fusions as indicated were spotted on synthetic complete medium lacking trytophan and leucine (SC-Leu-Trp). (Panel 2) Growth on the SC-Leu-Trp-His plate with 10 mM 3AT. Growth indicates an interaction between the two tested fusion proteins. (Panel 3) Growth on the SC-Leu-Trp-Ura plate containing 0.2% 5FOA. Poor or no growth on the SC-Leu-Trp-Ura plate with 0.2% 5FOA indicates interaction between two tested fusion proteins. (Panel 4) β-Galactosidase staining was performed on a nitrocellulose membrane as described in Materials and Methods. Dark staining indicates interaction between the two tested fusion proteins. β-Galactosidase activity was also measured by the Chlorophenol red-β-D-galactopyranoside (CPRG) cleavage assay and is indicated below the staining assay. The numbers shown are folds of increase in activity over the yeast strain that expressed GAL4-AD and GAL4-DB. Fulllength CEH-30 (wild type or F42E mutant) and UNC-37 proteins were fused to either the GAL4-AD or the GAL4-DB. GAL4-DB/GAL4-AD and GAL4-DB∷E2F1/GAL4-AD∷RB were used as negative and positive controls for protein interaction, respectively.