Abstract
The neurogenic regions such as subventricular zone of the lateral ventricles could become ischemic in some clinical situations due to the blockage of blood vessels by blood clots. Hence the aim of this study is to investigate the effects of OGD on the growth of neural progenitor cells and the phosphorylation of ERK, which plays an important role in the growth of these cells. Oxygen Glucose Deprivation (OGD) for 4 h decreased the growth of neural progenitor cells in vitro and also decreased the phosphorylation of ERK (extracellular signal regulated kinase). Inhibition of the ERK pathway for 4h using U0126 (10 μM) also decreased the growth of progenitor cells. These data suggest that a decline in the phospho-ERK content might decrease the growth of progenitor cells following OGD.
Keywords: Phosphorylation, OGD, ERK, Proliferation, Cell signaling
Neural progenitor cells are present in distinct regions of the brain proliferating in response to growth factors [1–4] and under pathological conditions [5, 6]. Several studies have demonstrated an increase in the proliferation of neural progenitor cells following cerebral ischemia [7–10]. It is interesting to note that most of the ischemic studies (Middle Cerebral Artery Occlusion) demonstrate damage to striatum and/or cortex. But, the neurogenic regions such as subventricular zone of lateral ventricles and subgranular zone of hippocampus harboring neural progenitor cells are not affected by ischemia. However, these neurogenic regions could be subjected to ischemia in some clinical situations due to the clogging of arteries by blood clots. Hence the aim of this study is to investigate the effects of OGD on the growth of neural progenitor cells in vitro. To understand the role of OGD in the growth of neural progenitor cells, we used cell proliferation assay [11]. Likewise we also studied the phosphorylation of extracellular signal regulated kinase (ERK) which plays an important role in the growth of cells [12], using phospho-specific antibodies and immunoblotting.
Adult rats (250–350g) were obtained from Charles River Laboratories and were cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Neural progenitor cells were cultured as previously described [13]. The animals were euthanized using high dose of isoflurane to minimize pain and the walls of lateral ventricles from adult rats were dissected, minced and digested with PPD solution containing (0.1% papain, 0.01% DNase, 0.1% Dispase) in Hanks balanced salt solution (HBSS). The dissociated cells (0.4 × 106) were cultured in neurobasal medium containing B27 supplement (without retinoic acid), FGF 20 ng/ml, heparin 2 μg/ml, glutamine (2 mM) and antibiotics for 5–7 days to generate neurospheres. The completely grown neurospheres were dissociated with accutase (sigma) and used for oxygen glucose deprivation studies. The dissociated progenitor cells were washed and incubated in OGD medium (20 mM NaHCO3, 120 mM NaCl, 5.36 mM KCl, 0.33 mM Na2HPO4, 0.44 mM KH2PO4, 1.27 mM CaCl2, 0.81 mM MgSO4) without glucose in an anaerobic chamber (PLAS LABS, Lansing, MI) containing nitrogen (85%), hydrogen (10%) and carbon dioxide (5%) mixture for 1–4 h [14]. Control cells were incubated in same medium containing glucose (5 mM) at 37°C in a regular CO2 (5%) incubator (Normoxic condition). For glucose deprivation, cells were incubated in OGD medium but incubated at 37°C in a CO2 incubator, while oxygen deprivation was achieved by adding glucose to OGD medium and incubating the cells in anaerobic chamber. In one set of experiments, cells were incubated in glucose containing medium in the presence of U0126 (10 μM), an ERK inhibitor, for 4 h at 37°C under normoxic conditions. Following the incubation at specific conditions, cells were either used for proliferation assay or immunoblotting.
Cells (1 × 104) were suspended in 100 μl neurobasal medium containing B27 (2%), heparin (2 μg/ml), FGF2 (20 ng/ml) in a 96 well plate. After 5 days in culture, 20 μl of assay reagent (CellTitre 96 AQueous; Promega Madison, WI) was added to the cell suspension and incubated at 37°C for 2 h to allow the conversion of tetrazolium salt into a formazan product. The optical density was measured at 490 nm in an ELISA plate reader. Culture medium without cells was used as blank. Before performing a cell proliferation assay, bright field images of the cells were captured using a Nikon camera. Following OGD, progenitor cells were homogenized in lysis buffer (20 mM Na2HPO4, pH 7.0, 50 mM NaF, 10 mM Na4P2O7, 150 mM NaCl, 5 mM EGTA, 5 mM EDTA, 2% Triton X-100, 1 mM Na3VO4 and 0.5% deoxycholate), centrifuged at 12,000 g for 10 min to clarify lysate and used for immunoblotting. 30 μg of protein was separated by poly acrylamide gel electrophoresis and transferred to a PVDF membrane. The membranes were blocked with 5% milk in Tris-buffered saline containing 0.1% Tween 20 followed by overnight incubation with primary antibodies for phospho-ERK1/2. Following washing, peroxidase-coupled secondary antibody (anti-mouse IgG) was added and incubated for 1 h. The membrane was washed and specific bands were visualized using super signal detection reagents (Pierce, Rockford, IL). The total protein content was normalized using mouse anti-actin antibodies (1:5,000). The intensity of the bands was measured using NIH image (SCION) software. The phosphorylation of ERK was expressed as the ratio of phospho-ERK to actin. Statistical analysis was performed by students “t” test and the results were compared to control. Results were considered to be significant if P< 0.05. Data is represented as mean ± S.D. of 3–5 experiments.
To study the effect of oxygen glucose deprivation (OGD) on the growth of progenitor cells, cultured neural progenitor cells were subjected to OGD in vitro, in an anaerobic chamber containing 85% nitrogen, 10% hydrogen and 5% carbon dioxide. OGD for 4 hours did not compromise the cell survival as analyzed by trypan blue staining, but decreased the growth of progenitor cells following replenishment of complete medium, as demonstrated by cell proliferation assay (Fig. 1). Results in Fig. 1 show an equal number of cells in normoxic and OGD conditions on day-1, but at the end of experimental period (5 days), the size of spheres decreased in the oxygen and glucose deprived cells. Oxygen glucose deprivation of neural progenitor cells for 4 hours decreased the phosphorylation of ERK in the adult neural progenitor cells (Fig. 2A). The decline in the phosphorylation of ERK following OGD was time dependent, beginning at 1h and continuing upto 4 h (Fig. 2B). Addition of glucose but not oxygen restored the phospho-ERK content (Fig. 2C). The growth of neural progenitor cells decreased following incubation in the presence of U0126, an ERK inhibitor, for 4 h (Fig. 3).
Fig. 1.
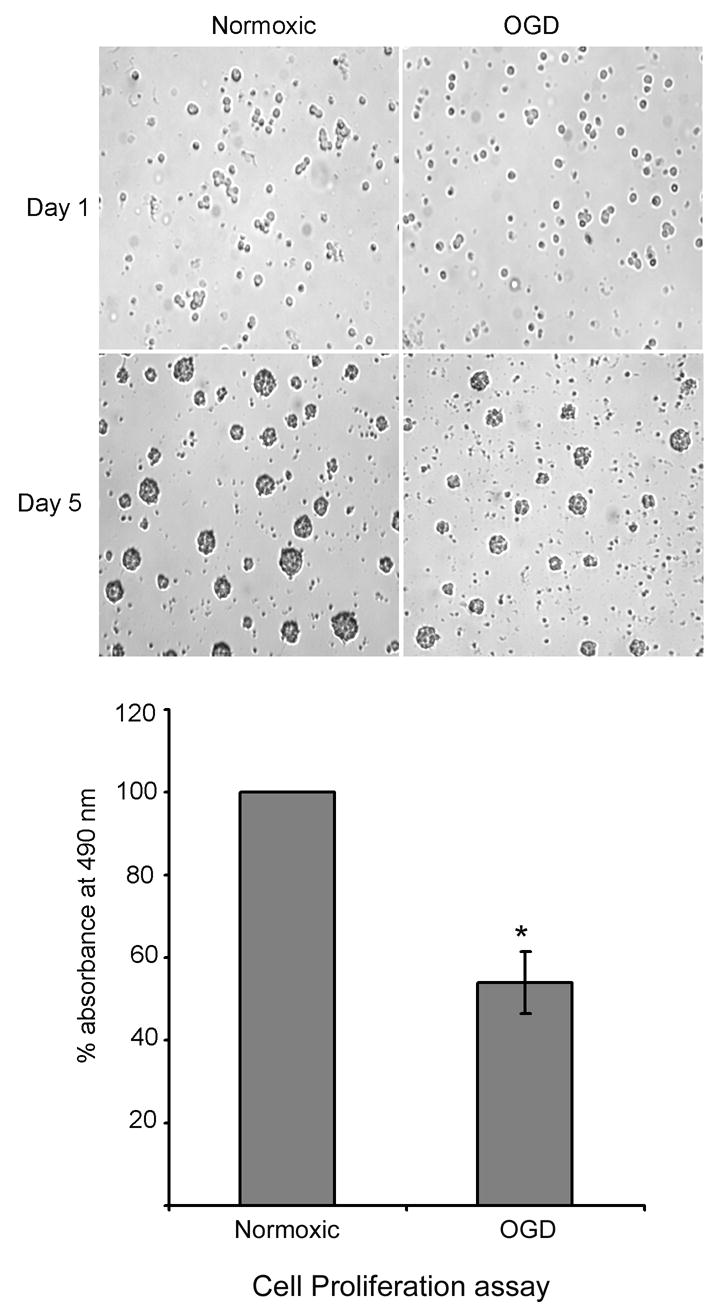
Effect of OGD on the proliferation of neural progenitor cells: Neural progenitor cells were subjected to OGD for 4 h, followed by culturing in neurobasal medium containing B27 and FGF2 for another 5 days. At the end of experimental period, cell proliferation assay was performed to detect the metabolic activity of the cells. The decrease in the metabolic activity of the cells is represented by bar graph. The cells were photographed before and after the experiment. * P < 0.05 as compared to normoxic cells.
Fig. 2.
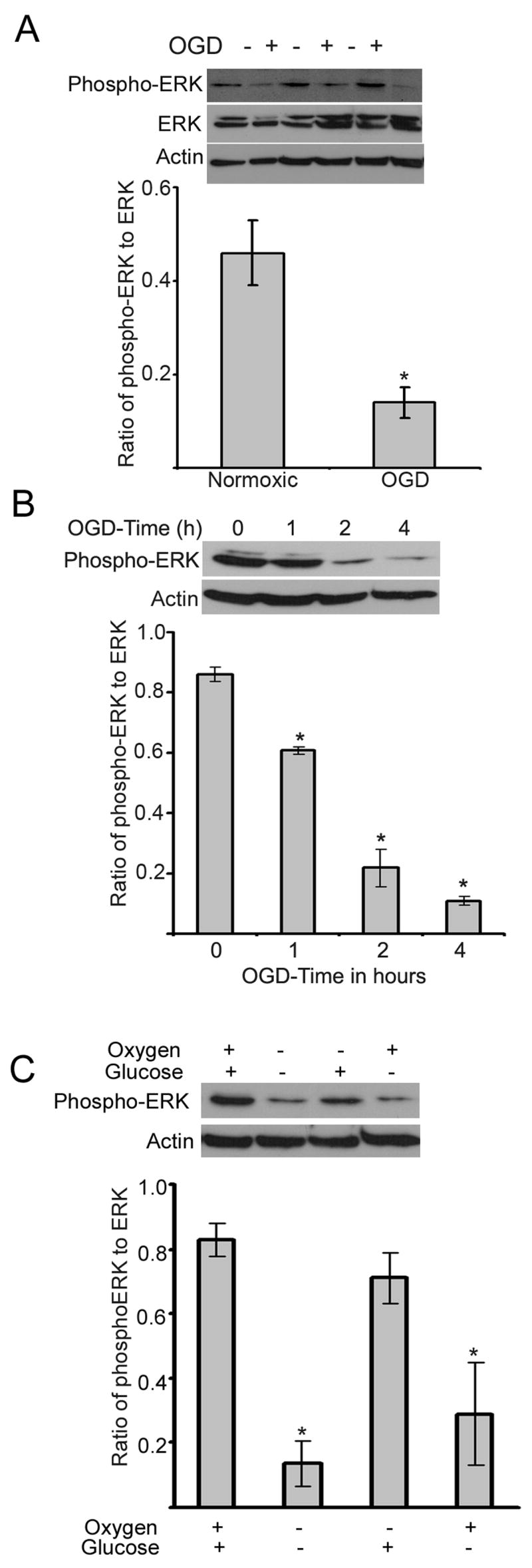
Effect of OGD on the phosphorylation of ERK. A: Neural progenitor cells were subjected to OGD for 4 h followed by lysis in lysis buffer. The lysate was subjected to immunoblotting using phospho-ERK, ERK and actin antibodies. Note: OGD decreased the phosphorylation of ERK but did not alter the content of ERK. * P < 0.05 as compared to normoxic cells. The samples are shown in triplicate.
B: Neural progenitor cells were subjected to OGD for various time points and the cell lysate was processed as described in Fig 2A. * P < 0.05 as compared to 0h incubation.
C: Effect of glucose and oxygen on the phosphorylation of ERK. Neural progenitor cells were subjected to OGD, or glucose deprivation or oxygen deprivation for 4 h and the phospho-ERK content was detected as described in Fig. 2A. Note: Oxygen but not glucose decreased the phosphorylation of ERK as compared to normoxic cells.
Fig. 3.
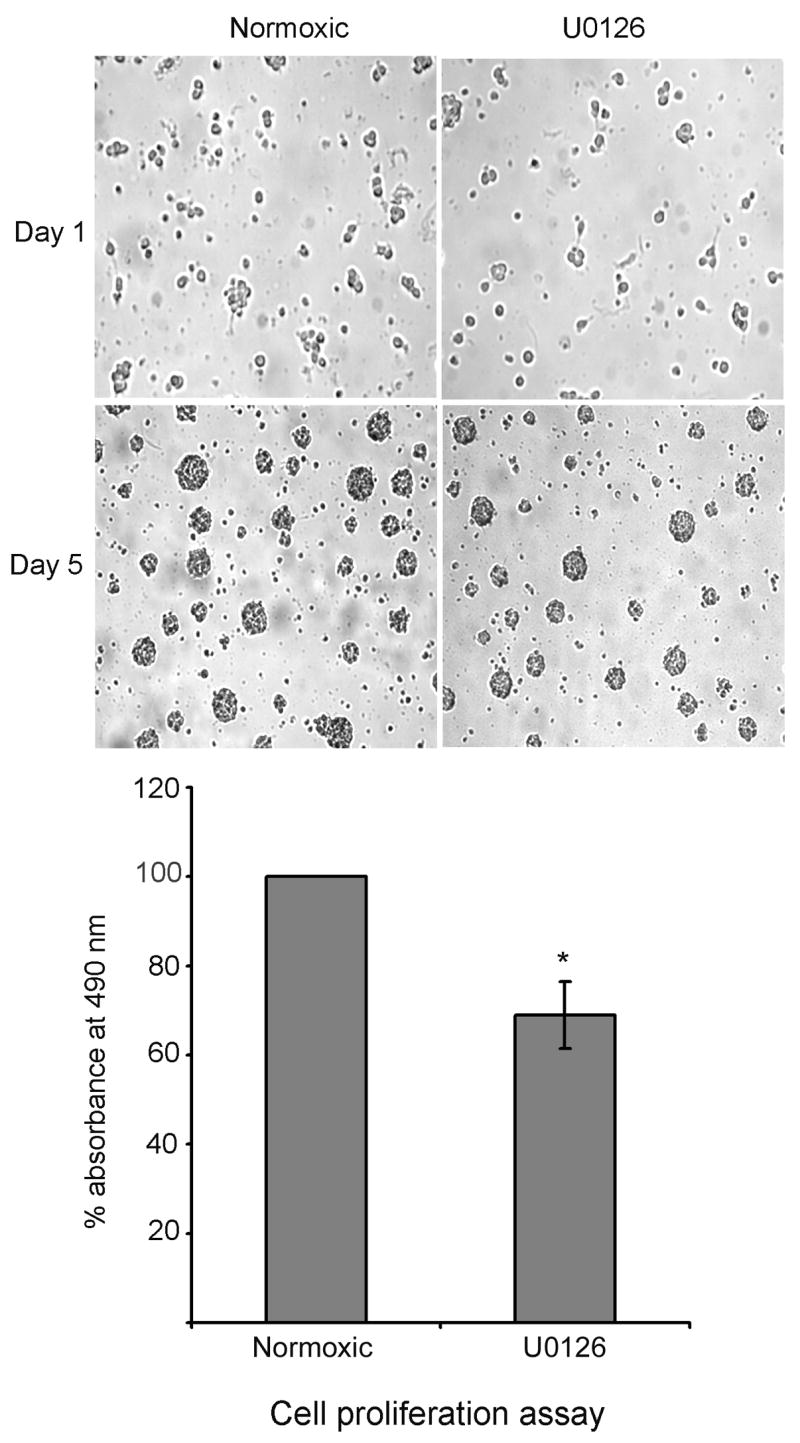
Inhibition of ERK pathway decreases the proliferation of neural progenitor cells. Neural progenitor cells were incubated under normoxic conditions in the presence and absence of U0125 (10uM) (ERK inhibitor) for 4 h. After the experimental period, cells were subsequently cultured in neurobasal medium containing B27 and FGF2 for 5 days. At the end of experimental period the cells were photographed and cell proliferation assay was performed as described in methods. * P < 0.05 as compared to normoxic cells.
Although the size of the neurospheres could not be measured precisely in free floating spheres, their metabolic activity was assessed by cell proliferation assay, an index for cell growth/proliferation. Our results demonstrate a decrease in the growth of cells following 4 h of OGD in vitro. It is very well known that OGD depletes the ATP content of the cells thus compromising with their metabolic activity. One of the important effects of ATP depletion is the alteration in the phosphorylation of proteins, which plays a major role in regulating the cellular activity. Phosphorylation of extracellular signal regulated kinase (ERK) is essential for the growth of neural progenitor cells [15]. Since OGD decreased the growth of cells, we studied the effect of OGD on the phosphorylation of ERK in adult neural progenitor cells. Our results illustrate a decrease in the phosphorylation of ERK following 4 h of OGD, and the decline in the phospho-ERK content was observed as a function of time (Fig 2B). These results are consistent with an earlier observation demonstrating a decline in the phosphorylation of kinases following OGD in astrocytes [16]. However, in their study, the phosphorylation of kinases increased at 2 hours and subsequently decreased during OGD. The discrepancy between their work and our observation could be due to the differences in the cell types (mature cells verses stem/progenitor cells).
To examine the role of glucose and oxygen in the phosphorylation of ERK, neural progenitor cells were exposed to either glucose or oxygen or both. Our results demonstrate the requirement of glucose, but not oxygen, for the phosphorylation of extracellular signal regulated kinase. One explanation for these results is the generation of ATP by anaerobic respiration in the presence of glucose and absence of oxygen resulting in the phosphorylation of proteins. Thus it appears that glucose may play an important role in the growth of cells. Our interpretation is consistent with an earlier observation which demonstrated that low glucose during proliferation decreased the proliferation of neural stem cells [17]. While oxygen deprivation decreased the growth in our work, previous studies have illustrated that low oxygen increased the proliferation of neural stem cells in vitro [18, 19].
To further understand the role of the ERK pathway in the growth of NPCs following OGD, we inhibited ERK using U0126 and studied the growth of NPCs. Consistent with a decrease in the phosphorylation of ERK and the growth of cells after OGD, inhibition of ERK using U0126 also decreased the growth of NPCs. These results indicate that inhibition of the ERK pathway during OGD may be one of the factors responsible for the decrease in the growth of NPCs. However, it should be noted that cell proliferation assay detects the metabolic activity of the cells but not the cell division. Indeed a decrease in the metabolic activity following OGD might result in the decrease in the growth of cells. Hence the oxygen and glucose deprived cells may be growing slowly as compared to normoxic cells due to the decrease in the metabolic activity. Thus it is logical to assume that OGD might decrease the rate of cell division.
These results together suggest that deprivation of oxygen and glucose in the neurogenic regions such as SVZ and DG during ischemia may decrease the proliferation of NPCs.
Acknowledgments
These studies were funded by NIH grant 5RO1NS045143-03 (RJD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer TD, Ray J, Gage FH. FGF-2-Responsive Neuronal Progenitors Reside in Proliferative and Quiescent Regions of the Adult Rodent Brain. Mol Cell Neurosci. 1995;6(5):474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 2.Martens DJ, Seaberg RM, vander Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16(6):1045–57. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 3.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20(8):2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14(3):237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172(1):115–127. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- 6.Mazarati A, Lu X, Shinmei S, Badie-Mahdavi H, Bartfai T. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience. 2004;128(2):431–441. doi: 10.1016/j.neuroscience.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87(3):586–97. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 10.Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri H, Vemuganti R, Dempsey RJ. Lack of response to epidermal growth factor in adult neural progenitor cells. Neuroreport. 2005;16(8):835–838. doi: 10.1097/00001756-200505310-00011. [DOI] [PubMed] [Google Scholar]
- 12.Harada J, Foley M, Moskowitz MA, Waeber C. Spingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88(4):1026–39. doi: 10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 13.Wachs FP, Couillard-Despres S, Engelhardt M, Wilhelm D, Ploetz S, Vroemen M. High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest. 2003;83(7):949–962. doi: 10.1097/01.lab.0000075556.74231.a5. [DOI] [PubMed] [Google Scholar]
- 14.Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+ exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci. 2005;25(49):11256–11268. doi: 10.1523/JNEUROSCI.3271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Learish RD, Bruss MD, Haak-Frendscho M. Inhibition of mitogen-activated protein kinase kinase blocks proliferation of neural progenitor cells. Brain Res Dev Brain Res. 2000;22(1):97–109. doi: 10.1016/s0165-3806(00)00064-x. [DOI] [PubMed] [Google Scholar]
- 16.Yung HW, Tolkovsky AM. Erasure of kinase phosphorylation in astrocytes during oxygen–glucose deprivation is controlled by ATP levels and activation of phosphatases. J Neurochem. 2003;86:1281–1288. doi: 10.1046/j.1471-4159.2003.01946.x. [DOI] [PubMed] [Google Scholar]
- 17.Horie N, Moriya T, Mitome M, Kitagawa N, Nagata I, Shinohara K. Lowered glucose suppressed the proliferation and increased the differentiation of murine neural stem cells in vitro. FEBS lett. 2004;571:237–242. doi: 10.1016/j.febslet.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 18.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced Proliferation, Survival, and Dopaminergic Differentiation of CNS Precursors in Lowered Oxygen. J Neurosci. 2000;20(19):7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20(19):7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


