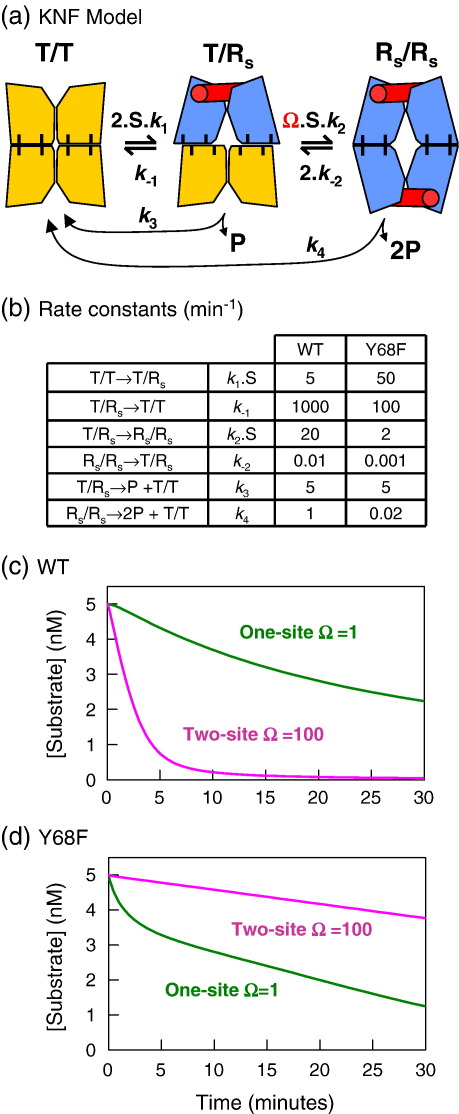
Fig. 8.
Mechanism for SfiI cooperativity. (a) The scheme indicates three conformational states: the free enzyme, T/T (T indicates the two subunits that form one DNA-binding unit, so the tetrameric protein is noted as T/T); the enzyme after a substrate-induced conformational change at one DNA-binding cleft, T/RS; the enzyme after induced changes in both clefts, RS/RS. The cartoons show the bound DNA as red cylinders and the subunits in the T and R states in yellow and blue, respectively. The T→RS transition is proposed to narrow the DNA-binding cleft and to increase the separation of the subunits at the dimer interface. The interactions between Tyr68 and Gln30 at this interface are indicated by hatch marks. Each step in this scheme is assigned a rate constant, as shown: statistical factors of 2 are applied to k1 and k− 2, to account for the two alternative routes for these steps, and a factor (Ω) of 100 is applied to k2[S] for reactions in cis. (b–d) The decrease in the concentration of intact DNA with time was calculated for the scheme in (a) by numerical integration using the value indicated in (b) for each rate constant. For all calculations, initial concentrations were [E0] = 0.5 nM and [S0] = 5 nM. These values yielded theoretical curves for the reactions of (c) wt SfiI on one-site DNA (green line) and two-site DNA (purple line), and (d) Y68F on one-site DNA (green line) and two-site DNA (purple line).