Supplementary Fig. 3.
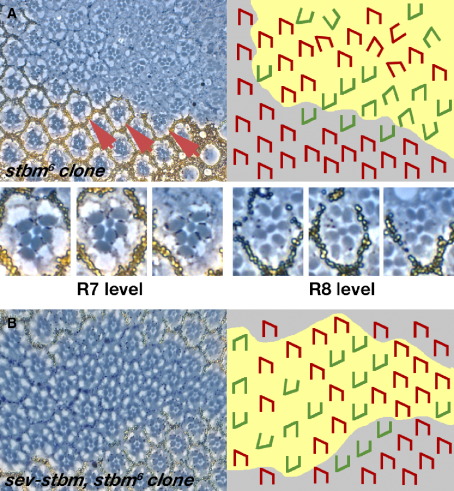
Non-autonomous phenotypes of stbm/Vang clones in the adult eye. Sections through adult eyes (dorsal up, posterior right) containing clones of cells homozygous for stbm6, marked by lack of pigment (brown). Cartoons on right show mutant tissue in yellow, dorsal-type ommatidia in red, ventral-type ommatidia in green. (A) stbm6 clone. Ommatidial polarity is randomised within the clone, with both rotation and dorsoventral chirality defects evident. On the equatorial edge of the clone (bottom edge in picture), inversions of ommatidial dorsoventral polarity are seen extending into wild-type tissue (arrows). Hence stbm/Vang exhibits both autonomous (within the clone) and non-autonomous (outside the clone) ommatidial polarity defects in the eye. Insets in lower part of panel show high magnification images of the three inverted ommatidia indicated by the arrows, taken from sections at both R7 and R8 photoreceptor level. Note dark pigment granules in all seven photoreceptors in the R7 level section, and in the stalk of the R8 photoreceptors (entering between R1 and R2) in the R8 level section, indicating that all 8 photoreceptors retain stbm/Vang activity. In total we examined 18 stbm6 clones and 16 stbm15 clones for evidence of non-autonomy of the stbm/Vang ommatidial polarity phenotype. Out of this set of 34 clones, we found 6 clones (3 each for stbm6 and stbm15) which had at least one ommatidium on the equatorial edge which was phenotypically mutant (i.e. showed inverted dorsoventral polarity) but genotypically wild-type for all 8 photoreceptors (9 ommatidia in total). Hence we conclude that stbm/Vang does show non-autonomy of the ommatidial polarity phenotype, albeit in only a relatively small subset of clones. This result stands in contrast to the findings of a previous analysis of the genotypes of at least 170 misoriented ommatidia on the edges of stbm/Vang clones, which concluded that the stbm/Vang polarity phenotype was autonomous (Wolff and Rubin, 1998; T. Wolff personal communication). We suggest two possible reasons for this discrepancy. Firstly, our data set might be more likely to reveal a low rate of ommatidial non-autonomy than the one previously analysed, by virtue of the way that we selected clones for analysis. As we were predisposed to the hypothesis that stbm/Vang clones might show equatorial non-autonomy of the polarity phenotype (by analogy to the proximal non-autonomy seen in the wing Taylor et al., 1998), we deliberately selected for analysis large clones with equatorial boundaries that were separated from the equator of the eye. Hence, even if stbm/Vang non-autonomy is only rarely seen, we will have greatly increased our chances of observing the phenomenon, as compared to the previously used data set in which misoriented ommatidia on all edges of clones in positions throughout the eye were examined (Wolff and Rubin, 1998). It should be noted that a low rate of ommatidial non-autonomy for the stbm/Vang phenotype is not unexpected, given that in the wing the range of non-autonomy around stbm/Vang clones only extends about 5–10 cells and varies depending upon clone position in the wing (Taylor et al., 1998; Adler et al., 2000). To see non-autonomy at the level of a whole ommatidium, all of the precursor cells for the 8 photoreceptors would have to lie within 5–10 cells of the equatorial edge of a stbm/Vang clone, a situation which would probably only occur rather infrequently. A second, less attractive, reason for the discrepancy may be that some of the stocks used to generate the clones have acquired either enhancers or suppressors of the stbm/Vang non-autonomous phenotype. We think this unlikely, as the chromosomes used in our study and the previous study were all generated in the same screen (Wolff and Rubin, 1998), and we see the non-autonomy with two different chromosomes—but we cannot rule out this possibility. (B) stbm6 clone in an eye also expressing stbm under control of the sevenless promoter, which is specifically expressed in differentiating photoreceptors. Expression of stbm under sevenless control is able to substantially rescue the ommatidial rotation defects within the clone, but not the dorsoventral polarity defects, suggesting that stbm/Vang has a function prior to photoreceptor differentiation which is required specifically to determine dorsoventral polarity. Note again that ommatidia with dorsoventral polarity defects are seen in wild-type tissue on the equatorial edge of the clone, indicating a non-autonomous role for stbm/Vang function in the eye.