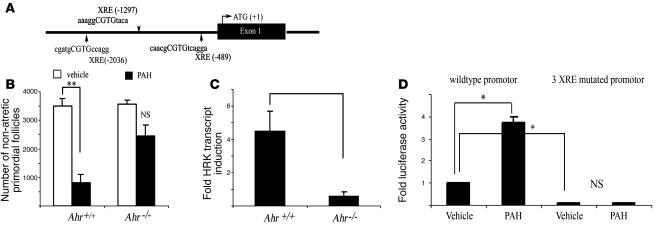
Figure 3. Ahr is involved in in vivo–induced follicle destruction in female offspring after maternal exposure to PAHs.
(A) Schematic outline of putative XRE elements, identified by a MatInspector computer algorithm within 5,000 bp of murine Hrk promoter. (B) Ahr-deficient offspring born to mothers exposed to PAHs prior to pregnancy and during lactation retained a significantly larger primordial follicle pool than their wild-type sibling sisters. Quantitative representation of the morphometric analysis of the ovarian pool for nonapoptotic oocyte-containing primordial follicles is expressed as mean ± SEM. Each genotype group represents combined data from offspring born to mothers exposed to vehicle (Ahr+/+, n = 4; Ahr–/–, n = 3) or treated with PAH (Ahr+/+, n = 7; Ahr–/–, n = 3). A significant reduction of follicles was only observed in the wild-type ovaries (**P = 0.0003). (C) Ahr-deficient neonatal ovaries failed to upregulate Hrk transcript expression in response to PAHs over a 24-hour period. The expression data are shown as fold change in PAH-treated ovary compared with vehicle-treated ovary from the same female (mean ± SEM) and were obtained from 4 sets of independent ovaries per treatment/genotype. PAH induced a significantly different response in wild-type and Ahr-deficient ovaries (P = 0.007). (D) PAH treatment (1 μM DMBA/BaP each) induces expression of luciferase driven by Hrk promoter in transient transfection assay using HEK cells (*P < 0.01). Activity of the promoter was greatly reduced by mutation of all 3 XRE sites in the presence of vehicle, but no induction occurred after PAH exposure using mutated construct. Data are shown as mean ± SEM of 3 independent experiments performed in pentuplicate and are expressed as fold change of luciferase/β-galactosidase ratio.